Abstract
In riparian forests, litter decay provides essential energy and nutrients for both terrestrial and fluvial ecosystems. Litter mixing effects (LMEs) are crucial in regulating litter decay and nutrient dynamics, yet how LMEs change over time is unclear in riparian forests. In this study, leaf litter of three common species (Alnus sibirica Fisch. ex Turcz, Betula platyphylla Sukaczev, and Betula fruticosa Pall.) were mixed in an equal mass ratio and LMEs were measured for mass and nitrogen (N) remaining in whole litter mixtures over a 3-year period in a boreal riparian forest, northeastern China. LMEs were also assessed for component litter mass and N remaining by separating litter mixtures by species. During the decay of litter mixtures, antagonistic effects on mass and N remaining were dominant after one and two years of decay, whereas only additive effects were observed after three years. LMEs correlated negatively with functional diversity after the first and two years of decay but disappeared after three years. When sorting litter mixtures by species, non-additive LMEs on mass and N remaining decreased over incubation time. Moreover, non-additive LMEs were more frequent for litter of both B. platyphylla and B. fruticosa with lower N concentration than for A. sibirica litter with higher N concentration. These results indicate that incubation time is a key determinant of litter mixing effects during decay and highlight that late-stage litter mixture decay may be predicted from single litter decay dynamics in boreal riparian forests.
Similar content being viewed by others
Introduction
In forests, litter decay regulates the formation of soil organic matter, nutrient cycling, and plant production (Prescott 2010; Kou et al. 2020). The rate of litter decay is primarily controlled by climate (Zhang et al. 2008; Canessa et al. 2022), litter quality variables (Xu et al. 2016; Zhang et al. 2019), and soil decomposers (Kominoski et al. 2011; Njoroge 2022). On the forest floor, litter of different species often mixes, producing litter mixing effects (LMEs) on decay. Previous studies have found that non-additive effects (i.e., the observed decay rate differs from the expected one) are more common than additive effects (i.e., the observed decay rate is equal to the expected one) (Hättenschwiler et al. 2005; Kou et al. 2020; Liu et al. 2020). Therefore, the prevalence of non-additive LMEs makes litter mixture decay unpredictable, based on monospecific litter decay in forests.
Litter mixture decay rates are reported to be faster (i.e., synergistic effects) or slower (i.e., antagonistic effects) than values calculated from monospecific litter decay rates (Hättenschwiler et al. 2005; Zhang et al. 2019; Liu et al. 2020). During mixed litter decay processes, synergistic effects are attributed to niche complementary among decomposers and interspecific litter nutrient transfer (Gessner et al. 2010; García-Palacios et al. 2017; Canessa et al. 2022), and antagonistic effects primarily result from the releases of inhibiting or recalcitrant organic compounds from component litter (Hättenschwiler et al. 2005; Zhang et al. 2019; Liu et al. 2020). Therefore, the diversity in litter chemical traits (i.e., functional diversity) is a powerful indicator that can be used to predict LMEs on decay and nutrient dynamics (Zhang et al. 2020; Swan and Sparkman 2023). Kou et al. (2020) observed that LMEs on litter decay are driven worldwide by litter functional diversity in forests. However, leaf litter quality is expected to converge during decay (Wallenstein et al. 2013). Accordingly, LMEs may decline gradually and even diminish when mixed litter decay proceeds. Unfortunately, empirical studies have seldom been conducted to assess how LMEs change over incubation time in forest soils (e.g., Lecerf et al. 2011; Singhal et al. 2021; Wu et al. 2022).
During litter mixture decay, the interspecific transfer of labile C and nutrients through leaching, diffusion, and fungal mycelia networks are possible mechanisms controlling the size of synergistic effects (Hättenschwiler et al. 2005; Gessner et al. 2010). For example, Bonanomi et al. (2014) found that mixing litter with different chemical properties produced synergistic effects on decay rates because of N transfer from N-rich to N-poor litter. Moreover, synergistic effects on decay are more frequent for plant litter with lower chemical qualities because the releases of labile C and nutrients from litter with higher chemical qualities stimulate microbial decay of low chemical quality litter (Wang et al. 2018; Liu et al. 2020). Therefore, clarifying the specific responses of component species in the litter mixtures should identify non-additive LMEs on litter decay rates. However, previous studies have often treated litter mixtures as a single entity, which does not clarify mixed litter decay rates and nutrient dynamics in forests.
Riparian forests act as an important source of energy and nutrients for adjacent terrestrial and aquatic ecosystems through plant litter decay, and thus play a critical role in maintaining ecosystem services at both watershed and regional scales (Kominoski et al. 2011; Riis et al. 2020). In these forests, altered species composition can influence litter mixture decay dynamics, which would lead to shifts in structure and function of both aquatic and terrestrial ecosystems. In this study, three woody species (Alnus sibirica Fisch. ex Turcz, Betula platyphylla Sukaczev, and Betula fruticosa Pall.) with contrasting leaf litter chemistry were selected in a boreal riparian forest in the north of Heilongjiang Province, northeastern China. A 3-year litterbag experiment was performed to measure mixed litter mass and N remaining during decay. Litter functional dispersion index was used to indicate functional diversity of litter mixtures (Laliberté and Legendre 2010). The aims were to determine how LMEs change with incubation time during decay and to clarify the behavior of component species within litter mixtures in boreal riparian forests. Therefore, two hypotheses were proposed: (1) non-additive LMEs were dominant in early stages due to functional diversity but decreased in the late stage; and (2) in the litter mixtures, synergistic effects of decay were more common for B. platyphylla and B. fruticosa than for A. sibirica because of lower leaf litter quality.
Materials and methods
Study site
This study was in a boreal riparian forest near the Emuer River in Heilongjiang Province, northeastern China (52°56′ N, 122°51′ E) located in a region of continuous permafrost and a temperate continental monsoon climate. Average annual precipitation and annual air temperature are 450 mm and − 3.9 ℃, respectively. In the riparian forest, the dominant tree species are A. sibirica and B. platyphylla, and the accompanying species are Chosenia arbutifolia (Pall.) A. Skv, Populus davidiana Dode, and Rhamnus davurica Pall.. The main understory shrubs are B. fruticosa and Salix rosmarinifolia L. The ground is covered by a dense litter layer and sedges and mosses. Organic C, total nitrogen (N), and total phosphorus (P) in the forest floor are 450.0, 70.2, and 6.7 mg g−1, respectively.
Litter decay experiment
To create a gradient of litter functional diversity, leaf litter of an N2-fixing deciduous tree (A. sibirica) and two non-N2-fixing deciduous trees (B. platyphylla and B. fruticosa) were selected. In August 2012, five 15 × 15 m plots were selected and freshly fallen leaves without obvious symptoms of herbivory were collected using litter traps. Litter samples were grouped into species based on morphological traits, oven-dried to constant mass, and stored in plastic bags. A portion was milled (< 0.15 mm sieve) and analyzed for organic C content with a TOC analyzer (Multi N/C 2100, Analytik Jena, Germany), for total N and P concentrations with a continuous-flow analyzer (AA3, Seal Analytical, Germany) after the Kjeldahl digestion (Graça et al. 2005), and for lignin with a fiber analyzer (FT350, FOSS, Denmark) with the gravimetric method (Möller 2009).
In this study, there were three monospecific litters (B. platyphylla, A. sibirica, and B. fruticosa) and four mixed litters (B. platyphylla + A. sibirica, B. platyphylla + B. fruticosa, A. sibirica + B. fruticosa, and B. platyphylla + A. sibirica + B. fruticosa). In the mixed litter, component species were mixed by equal mass ratio. For both monospecific and mixed litters, 6-g oven-dried litter were placed in 30 × 25 cm nylon litterbags (1 mm mesh size). The litterbags were buried in the litter layer in each plot and collected after one, two, and three years. There were three sampling dates and five replicates, providing 15 litterbags per treatment. For monospecific litters, litter was sampled from the litterbags, cleaned with deionized water, dried to constant weight, and weighed.
The remaining litter mixtures were separated into component species according to litter morphology and toughness, and treated with the same procedures as monospecific litter. Litter per treatment was ground (< 0.15 mm) and used to determine N concentration as described. On each sampling date, litter mass remaining was obtained on the basis of the initial and final mass. In addition, litter N remaining was obtained from litter mass remaining and N concentration.
Statistical analysis
Community-weighted chemical traits of litter mixtures were obtained from initial chemical traits and mixing proportion within litter mixtures. Litter functional diversity was expressed as litter functional dispersion index (FDis), which shows the average distance of each component litter to the community-weighted centroids of litter mixtures (Laliberté and Legendre 2010). Based on seven initial litter chemical traits (organic C, total N, total P, and lignin and C:N, C:P, and lignin:N ratios). FDis were calculated on a species − species distance matrix (i.e., Gower dissimilarity matrix) using the FD package in R 4.1.1 (Laliberté and Legendre 2010). Litter chemical traits are shown in Table 1.
For both mass and N remaining, the expected value of whole litter mixtures (\({{\delta }_{\mathrm{Remaining}}}_{\mathrm{exp}}\)) was calculated by (Bonanomi et al. 2014; Zhang et al. 2020):
where \({{\delta }_{\mathrm{Remaining}}}_{\mathrm{obsi}}\) was the observed value of monospecific litter i and \({{\delta }_{\mathrm{Pro}}}_{i}\) was the mixing mass ratio of monospecific litter i. LMEs on mass and N remaining of whole litter mixtures (\({\delta }_{\mathrm{LMEsw}}\)) were obtained by (Bonanomi et al. 2014; Zhang et al. 2020):
where \({{\delta }_{\mathrm{Remaining}}}_{\mathrm{obs}}\) was the observed mass or N remaining of whole litter mixtures. LMEs on mass and N remaining of component species within litter mixtures (\({\delta }_{\mathrm{LMEsc}}\)) were obtained by (Wu et al. 2022):
where \({{\delta }_{\mathrm{Remaining}}}_{\mathrm{mixi}}\) was the mass or N remaining of component species i during decay within the litter mixtures.
For both whole litter and component mixtures, the significant difference between LMEs and zero was assessed with a one-sample t test. Based on the statistical results, LMEs were subdivided into additive effects when LMEs did not differ from zero, synergistic effects when LMEs were greater than zero, and antagonistic effects when LMEs were lower than zero (Hättenschwiler et al. 2005; Gessner et al. 2010).
Statistical analyses were performed in R 4.1.1 (R Development Core Team 2021), and P < 0.05, P < 0.01, and P < 0.001 were considered statistically significant, very statistically significant, and extremely statistically significant, respectively. Linear mixed models (LMMs) were adopted to examine the impacts of species and incubation time on monospecific litter mass and N remaining. At each incubation time, Tukey’s HSD test was performed to detect differences in litter mass and N remaining among species. LMMs were carried out to test the effects of litter type and incubation time on LMEs during the decay process. Pearson’s correlation analysis tested the correlation between LMEs and litter functional diversity during the decay of whole litter mixtures.
Results
Monospecific leaf litter decay
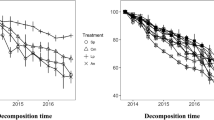
Litter type and incubation time significantly influenced litter mass remaining (P < 0.001, Fig. 1). Incubation time, litter type, and their interaction significantly affected litter N remaining (P < 0.001, Fig. 1). Across the incubation periods, both A. sibirica and B. fruticosa had lower litter mass remaining than B. platyphylla (Fig. 1). Both B. platyphylla and B. fruticosa had higher litter N remaining than A. sibirica (Fig. 1).
Monospecific leaf litter mass and N remaining over three-year decay in a boreal riparian forest in northeastern China. Error bars are the standard errors of the means (n = 5). At each incubation time, different lowercase letters indicate the significant difference among monospecific litter treatments at P < 0.05
LMEs during the decay process of litter mixtures
Mixed litters, incubation time, and their interaction significantly affected LMEs during decay (P < 0.01, Fig. 2). After one- and two-year decay periods, antagonistic effects were more common than additive and synergistic effects for both mass and N remaining of whole litter mixtures (Fig. 2). However, only additive effects were observed for mass and N remaining of whole litter mixtures after three-year decay (Fig. 2). LMEs showed a significant negative relationship with litter functional diversity after one- and two-year decay (P < 0.01) but exhibited no significant relationship with functional diversity after three-year decay (Fig. 3).
Litter mixing effects on mass and N remaining of the whole litter mixtures over three-year decay in a boreal riparian forest in northeastern China. Error bars are the standard errors of the means (n = 5). For each litter mixture, ns, *, **, and *** indicate the significant difference between litter mixing effects and zero at P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively
The Pearson’s correlation analysis showing the relationships between litter mixing effects and functional diversity of the whole litter mixtures over three-year decay in a boreal riparian forest in northeastern China. At each incubation time, ns and ** indicate the significant difference between litter mixing effects and zero at P > 0.05 and P < 0.01, respectively
Component litter decay within litter mixtures
During the decay of litter mixtures, litter N concentration of B. platyphylla and B. fruticosa initially increased but subsequently declined, whereas A. sibirica litter N concentration declined with longer periods of decay (Fig. 4). Within litter mixtures, A. sibirica litter often produced antagonistic effects on mass remaining of the neighboring litter, although its mass remaining was barely affected over three years of decay (Fig. 5). In the litter mixtures, A. sibirica litter often produced synergistic effects on neighboring litter N remaining, but antagonistic effects were predominant for N remaining of A. sibirica litter after one and two years (Fig. 5). After three years of decay, the additive effects on component litter N remaining occurred in the litter mixtures containing A. sibirica (Fig. 5). Moreover, additive effects were observed for component litter mass and N remaining over three years for B. platyphylla + B. fruticosa litter mixture (Fig. 5).
Litter mixing effects on mass and N remaining of component species in the litter mixtures over three-year decay in a boreal riparian forest in northeastern China. Error bars are the standard errors of the means (n = 5). For each component species, ns, *, **, and *** indicate the significant difference between litter mixing effects and zero at P > 0.05, P < 0.05, P < 0.01, and P < 0.001, respectively
Discussion
In agreement with the first hypothesis, non-additive effects, especially antagonistic effects, were predominant for litter mass and N remaining in early-stage decay (i.e., the initial two years), but disappeared in late-stage decay (i.e., after three years). Previous studies have also shown that synergistic effects changed to additive or antagonistic effects as litter mixture decay in shrublands and forests proceeded (Santonja et al. 2019; Singhal et al. 2021; Wu et al. 2022). These results imply that incubation time regulates the direction of LMEs on litter decay in forests. In the early stage, the release of soluble organic matter fractions from litter could facilitate labile C and nutrient transfers among litter components via diffusion and fungal mycelia and enhance complementary resource use among decomposers, which would account for the synergistic effects of litter decay (Hättenschwiler et al. 2005; García-Palacios et al. 2017; Liu et al. 2020). When decay proceeds, labile organic compounds are exhausted by soil microbes, and recalcitrant organic compounds (e.g., lignin and condensed tannins) remain in the decomposing litter (Wang et al. 2022). Thus, plant litter qualities often converge (Fig. 4), which reduces the synergistic effects of mixed litter decay (Kou et al. 2020). Moreover, the enriched recalcitrant organic compounds in decomposing litter over time suppress microbial activity and thus inhibit decay of litter mixtures (Mortensen et al. 2019; Wang et al. 2022). Accordingly, additive effects were observed after three years of litter decay. Overall, the temporal shift in LMEs from non-additive to additive effects highlights that the decay rates of litter mixtures can be ultimately projected based on the single litter decay dynamics in boreal riparian forests.
During early-stage decay, LMEs on mass and N remaining changed from additive to antagonistic effects with increasing functional diversity among component species. Several studies have shown that higher chemical diversity often yields greater LMEs on mixed litter decay in forests (Zhang et al. 2020; Canessa et al. 2022). A large diversity of litter chemical traits within litter mixtures could create nutrient gradients among component species and produce diverse substrates for decomposers, which would account for two possible mechanisms (i.e., nutrient transfer and niche complementary hypotheses) driving the non-additive LMEs (Bonanomi et al. 2014; García-Palacios et al. 2017; Swan and Sparkman 2023). In this study, A. sibirica, an N2-fixing tree species, had higher litter N and lignin concentrations but had lower litter P concentration than B. platyphylla and B. fruticosa (Table 1). Thus, the presence of A. sibirica litter can enhance functional diversity of litter mixtures, increasing the occurrence of non-additive LMEs in the early stage of mixed litter decay. These findings suggest that litter chemical diversity is a powerful indicator of LMEs on early-stage litter decay in forest ecosystems.
During monospecific litter decay, A. sibirica had lower N remaining than the other two species but had comparable litter mass remaining to B. fruticosa. These different interspecific patterns imply that litter decay differs from litter N release during decay. Litter decay rates are limited by labile organic C fractions and nutrients in the early stage, but are inhibited by recalcitrant organic C factions such as lignin in the late stage (Zhang et al. 2008; Prescott 2010). Among the selected three species, A. sibirica had the highest N and lignin concentrations, whereas B. fruticosa had the lowest lignin concentration. Thus, A. sibirica had comparable litter mass remaining with B. fruticosa, but had higher litter mass remaining than B. platyphylla across three years of decay. In contrast, litter N dynamic is primarily controlled by its C:N ratio during decay (Manzoni et al. 2010). Due to lower litter C:N ratio, A. sibirica always had higher litter N release than B. platyphylla and B. fruticosa during decay. In boreal riparian forests, the abundance of alders is observed to decline because of fungal pathogens (Alonso et al. 2022) or to increase due to global change (Hiltbrunner et al. 2014). Given the interspecific variation of litter chemistry between alders and non-N2-fixing trees, such alteration of vegetation composition will strongly influence litter decay and soil nutrient availability in boreal riparian forests.
During the initial two-year decay of litter mixtures, synergistic effects were stronger for B. platyphylla and B. fruticosa than for A. sibirica. Accordingly, our results partly supported the second hypothesis. Wang et al. (2018) also showed that mixing low-quality coniferous litter with high-quality broadleaf litter accelerated coniferous litter decay in subtropical plantations. During litter mixture decay, nutrient transfer could alleviate the nutrient limitation of decomposers and thus stimulates the decay of low-quality litter (Bonanomi et al. 2014; Liu et al. 2020). In the present study, the declined litter N concentration of A. sibirica together with the increased litter N concentration of B. platyphylla and B. fruticosa (Fig. 4) further supported that N-rich A. sibirica litter acted as a net N source for the neighboring N-poor litter during early-stage decay. When mixed litter decay proceeded, litter N concentration of component species became convergent (Fig. 4). Thus, additive effects were dominant for low-quality litter after three years of decay. These results regarding the different behaviors between high-quality and low-quality litter confirm that nutrient transfer among component species should be a key determinant of synergistic effects during mixed litter decay in forests. However, we only measured litter total N concentration in the present study, which could not discriminate the sources of N in component litter. Therefore, in further studies, 15N-labeled leaf litter should be used to identify the N sources of component litter, which can effectively uncover the role of nutrient transfer in driving LMEs during litter mixture decay in forests.
Conclusions
During the three-year decay of litter mixtures, LMEs on litter decay and nutrient release changed from synergistic effects to additive effects over incubation time in a boreal riparian forest. The significant relationship between LMEs and litter functional diversity was only observed in the initial two-year decay of litter mixtures. Furthermore, non-additive LMEs were more common for low-quality litter species (B. platyphylla and B. fruticosa) than for high-quality litter species (A. sibirica). In summary, the temporal variation of LMEs over incubation time highlights that, despite the complex species interactions during early-stage decay, late-stage litter mixture decay can be easily projected from single litter decay dynamics in boreal riparian forests.
References
Alonso A, López-Rojo N, Pérez J, Boyero L (2022) Functional consequences of alder and oak loss in stream ecosystems. Freshwater Biol 67:1618–1630. https://doi.org/10.1111/fwb.13965
Bonanomi G, Capodilupo M, Incerti G, Mazzoleni S (2014) Nitrogen transfer in litter mixture enhances decomposition rate, temperature sensitivity, and C quality changes. Plant Soil 381:307–321. https://doi.org/10.1007/s11104-014-2119-4
Canessa R, van den Brink L, Berdugo MB, Hättenschwiler S, Rios RS, Saldaña A, Tielbörger K, Bader MY (2022) Trait functional diversity explains mixture effects on litter decomposition at the arid end of a climate gradient. J Ecol 110:2219–2231. https://doi.org/10.1111/1365-2745.13946
García-Palacios P, Shaw EA, Wall DH, Hättenschwiler S (2017) Contrasting mass-ratio vs. niche complementarity effects on litter C and N loss during decomposition along a regional climatic gradient. J Ecol 105:968–978. https://doi.org/10.1111/1365-2745.12730
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S (2010) Diversity meets decomposition. Trends Ecol Evol 25:372–380. https://doi.org/10.1016/j.tree.2010.01.010
Graça MAS, Bärlocher F, Gessner MO (2005) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, pp 53–60
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Ann Rev Ecol Evol Syst 36:191–218. https://doi.org/10.1146/annurev.ecolsys.36.112904.151932
Hiltbrunner E, Aerts R, Bühlmann T, Huss-Danell K, Magnusson B, Myrold DD, Reed SC, Sigurdsson BD, Körner C (2014) Ecological consequences of the expansion of N2-fixing plants in cold biomes. Oecologia 176:11–24. https://doi.org/10.1007/s00442-014-2991-x
Kominoski J, Marczak LB, Richardson JS (2011) Riparian forest composition affects stream litter decomposition despite similar microbial and invertebrate communities. Ecology 92:151–159. https://doi.org/10.1890/10-0028.1
Kou L, Jiang L, Hättenschwiler S, Zhang MM, Niu SL, Fu XL, Dai XQ, Yan H, Li SG, Wang HM (2020) Diversity-decomposition relationships in forests worldwide. eLife 9:e55813. https://doi.org/10.7554/eLife.55813
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Lecerf A, Marie G, Kominoski JS, LeRoy CJ, Bernadet C, Swan CM (2011) Incubation time, functional litter diversity, and habitat characteristics predict litter-mixing effects on decomposition. Ecology 92:160–169. https://doi.org/10.1890/10-0315.1
Liu J, Liu XY, Song QN, Compson ZG, LeRoy CJ, Luan FG, Wang H, Hu YL, Yang QP (2020) Synergistic effects: a common theme in mixed-species litter decomposition. New Phytol 227:757–765. https://doi.org/10.1111/nph.16556
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. https://doi.org/10.1890/09-0179.1
Möller J (2009) Gravimetric determination of acid detergent fiber and lignin in feed: interlaboratory study. J AOAC Int 92:74–90. https://doi.org/10.1093/jaoac/92.1.74
Mortensen LH, Cruz-Paredes C, Schmidt O, Rønn R, Vestergård M (2019) Ash application enhances decomposition of recalcitrant organic matter. Soil Biol Biochem 135:316–322. https://doi.org/10.1016/j.soilbio.2019.05.021
Njoroge DM, Chen SC, Zuo J, Dossa GGO, Cornelissen JHC (2022) Soil fauna accelerate litter mixture decomposition globally, especially in dry environments. J Ecol 110:659–672. https://doi.org/10.1111/1365-2745.13829
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149. https://doi.org/10.1007/s10533-010-9439-0
R Development Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Riis T, Kelly-Quinn M, Aguiar FC, Manolaki P, Bruno D, Bejarano MD, Clerici N, Fernandes MR, Franco JC, Pettit N, Portela AP, Tammeorg O, Tammeorg P, Rodríguez-González PM, Dufour S (2020) Global overview of ecosystem services provided by riparian vegetation. Bioscience 70:501–514. https://doi.org/10.1093/biosci/biaa041
Santonja M, Milcu A, Fromin N, Rancon A, Shihan A, Fernandez C, Baldy V, Hättenschwiler S (2019) Temporal shifts in plant diversity effects on carbon and nitrogen dynamics during litter decomposition in a Mediterranean shrubland exposed to reduced precipitation. Ecosystems 22:939–954. https://doi.org/10.1007/s10021-018-0315-4
Singhal V, Roy T, Singh C, Ghosh J (2021) Effect of incubation time, litter diversity and species richness on decomposition dynamics of tree species from western Himalayas. CATENA 203:105281. https://doi.org/10.1016/j.catena.2021.105281
Swan CM, Sparkman A (2023) The role of functional and phylogenetic diversity in riparian tree vegetation on leaf litter breakdown in rivers. Oikos 2023:e09361. https://doi.org/10.1111/oik.09361
Wallenstein MD, Haddix ML, Ayres E, Steltzer H, Magrini-Bair KA, Paul EA (2013) Litter chemistry changes more rapidly when decomposed at home but converges during decomposition-transformation. Soil Biol Biochem 57:311–319. https://doi.org/10.1016/j.soilbio.2012.09.027
Wang H, Liu SR, Wang JX, You YM, Yang YJ, Shi ZM, Huang XM, Zheng L, Li ZY, Ming AG, Lu LH, Cai DX (2018) Mixed-species plantation with Pinus massoniana and Castanopsis hystrix accelerates C loss in recalcitrant coniferous litter but slows C loss in labile broadleaf litter in southern China. Forest Ecol Manag 422:207–213. https://doi.org/10.1016/j.foreco.2018.04.024
Wang LF, Zhou Y, Chen YM, Xu ZF, Zhang J, Liu Y, Joly F (2022) Litter diversity accelerates labile carbon but slows recalcitrant carbon decomposition. Soil Biol Biochem 168:108632. https://doi.org/10.1016/j.soilbio.2022.108632
Wu PP, Jiang LX, Zhang Y, Tu QH, Mao R (2022) Manganese addition accelerates litter decomposition and alters litter mixing effects in the late stage in subtropical plantations of southern China. Plant Soil 481:501–510. https://doi.org/10.1007/s11104-022-05652-6
Xu ZF, Zhu JX, Wu FZ, Liu Y, Tan B, Yang WQ (2016) Effects of litter quality and climate change along an elevational gradient on litter decomposition of subalpine forests, Eastern Tibetan Plateau, China. J For Res 27:505–511. https://doi.org/10.1007/s11676-015-0180-3
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. https://doi.org/10.1093/jpe/rtn002
Zhang XX, Wang BY, Liu ZW (2019) Impacts of plant secondary metabolites from conifer litter on the decomposition of Populus purdomii litter. J For Res 30:2237–2245. https://doi.org/10.1007/s11676-018-0766-7
Zhang XH, Wang L, Jiang W, Mao R (2020) Functional identity and functional diversity co-regulate litter mixture decomposition and nitrogen release in boreal riparian forest ponds. Biogeochemistry 151:99–111. https://doi.org/10.1007/s10533-020-00716-0
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This research was funded by National Natural Science Foundation of China (41771108 and 31570479), the Natural Science Foundation of Jiangxi, China (20212ACB215002), and the Double Thousand Plan of Jiangxi Province (jxsq2018106044).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Liu, B., Li, R. et al. Temporal changes in mixing effects on litter decay and nitrogen release in a boreal riparian forest in northeastern China. J. For. Res. 35, 1 (2024). https://doi.org/10.1007/s11676-023-01662-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11676-023-01662-x