Abstract
In southern China, the eucalyptus plantation industry has been severely restricted by government policy over concerns on negative environmental impacts. In its place, large-scale plantations of high-value tropical tree species such as nitrogen-fixing Dalbergia odorifera and hemiparasite Santalum album have been widely cultivated including in mixed-species plantations. However, despite their poor growth, little information is available on suitable silvicultural practices of these plantations. Therefore, we subjected an 8-year-old mixed stand of D. odorifera and S. album to weeding, fertilization, weeding + fertilization, or no (CK) treatments and measured soil microbial biomass, respiration, nutrients, nitrogen mineralization and leaching and tree growth and litter production. Weeding and fertilization decreased microbial biomass but increased soil respiration, inhibited mineralization, had not effect on leaching of soil nitrogen, and improved the nutrient status of plantation soil. All practices improved the growth of D. odorifera. In the mixed plantation, fertilization increased litter production and nutrient content, but weeding and weeding + fertilization decreased growth of S. album and litter production in mixed plantation because weeding decreased the number of S. album haustoria in underground plant roots. In conclusion, fertilization is recommended; however, weeding-related practices are inappropriate for D. odorifera and S. album mixed plantations. These conclusions have important implications for managing other parasite or mixed-species plantations.
Similar content being viewed by others
Introduction
Management practices are applied to improve the production and quality of plantation forests by increasing soil nutrient availability and regulating competition among plants (Erb et al. 2018; Sida et al. 2018). Weeding and fertilization are the most common of these practices around the world for tree species such as Populus spp. (Pokharel and Chang 2016), Eucalyptus spp. (Carrero et al. 2018), Pinus banksiana (Pokharel et al. 2017), Cunninghamia lanceola (Wang et al. 2008) and Phyllostachys edulis (Li et al. 2016; Song et al. 2020).
Biological and biochemical processes in the soil such as microbial activity and nutrient transformation contribute to maintaining soil ecological functions. Weeding changes energy and nutrient inputs into the soil by decreasing vegetation cover in plantations (Rey et al. 2011; Zhang et al. 2018). Thus, weed affects not only soil physicochemical properties, such as temperature, water content (Özkan and Gökbulak 2017), pH (Li et al. 2014), and nutrient availability (Rey et al. 2011), but also the quantity and activity of roots and microbes in the soil (Fierer et al. 2012; Allison et al. 2013).
Fertilization is commonly used to increase nutrient content and availability in the soils and thus improve productivity of plantations (Fox 2000). It also can affect soil biological and biochemical properties processes such as soil microbial biomass, nutrient cycling and respiration rate (Lee and Shibu 2003). Numerous studies have explored the effects of fertilizer application, especially nitrogen (N), on soil biological and biochemical processes, but results have been inconsistent; fertilizer application has significantly increased soil microbial biomass (Li et al. 2010; Song et al. 2020), microbial community diversity (Ramirez et al. 2010) and soil respiration rate (Bowden et al. 2004) and also had negative or neutral effects (Samuelson et al. 2009; Sun et al. 2011; Wang et al. 2017). The inconsistencies can be due to differences in tree species, plantation age, site conditions and fertilizer content and dosage (Peng et al. 2008). In addition, little is known about the effects of combined weeding and fertilization on plantation ecosystems. A better understanding of these effects will help better manage plantation ecosystem functions.
In southern China, concerns about negative environmental impacts from eucalyptus plantation industry have led to severe governmental policy restrictions. Instead, high-value tropical tree species such as Dalbergia odorifera and Santalum album have been widely grown in large-scale mixed-species plantations. Both species are renowned for their valuable heartwood and widely used as religious, cosmetic, furniture and medicinal materials (Dhanya et al. 2010; Cui et al. 2019). Santalum species are hemiparasites that take up water and nutrients from host plants through haustoria in the roots (Lu et al. 2014). In addition, D. odorifera is a good host for S. album because it is strong nitrogen-fixer (Lu et al. 2017). However, little information is available on suitable cultivation practices for these plantations (Cui et al. 2017). Furthermore, herbicides must be replaced by manual weeding to avoid harming S. album because of its parasitic characteristics, and intensive farming and fertilization must be implemented in place of extensive traditional management practices. Weeding promotes growth of trees by minimizing neighboring competition, or it may inhibit the growth of S. album by decreasing the number of S. album haustoria in plant roots. However, little is known about the mechanism by which weeding and fertilization regulate ecosystem functions in mixed stands of D. odorifera and S. album. A better mechanistic understanding of these management practices will help manage forest plantations more effectively.
A weeding and fertilization experiment was conducted in a mixed-species plantation of D. odorifera and S. album to study changes in soil microbial biomass, respiration, nutrients, tree growth and litter production. We examined (a) whether weeding decreases and fertilization increase soil microbial biomass and soil respiration, (b) whether weeding and fertilization improve soil nutrients and N transformation, and (c) whether a combined treatment of weeding and fertilization promotes growth of trees over the single and the control treatments.
Materials and methods
Site description
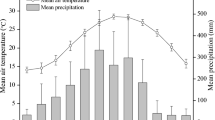
At the experimental plantation in Foshan City, Guangdong, China (22°47´N, 112°32´E), mean annual precipitation is 1681 mm and mean air temperature is 23.4 °C. The dry season from October to March received 18% of the annual precipitation during the study period (Fig. 1).
The mixed-species plantation of D. odorifera and S. album in the study was established in 2009 with a species ratio of 1:1, alternately planted with a spacing 2.5 m × 2.5 m. At the beginning of treatments in April 2017, the survival rate was ~ 94% (750 trees/ha) for D. odorifera and ~ 88% (700 trees ha−1) for S. album. At 8 years of age, D. odorifera had a mean DBH of 7.63 ± 1.46 cm and mean height of 6.29 ± 0.87 m and S. album had a mean DBH of 7.04 ± 1.51 cm a mean height of 5.53 ± 0.86 m.
The soil in the mixed plantation is classified as haplic acrisols according to the FAO soil classification. Initial data for soil status are shown in Table 1.
Experimental design and sampling
The experiment was laid out in a randomized complete block design with four treatments (control [CK], weeding [W], fertilization [F], and weeding + fertilization [W + F]) and four replicates. Each of the 16 replicate treatment plots was 400 m2. For the weeding treatment, ground vegetation was removed manually with a spade and spread evenly on the ground. For the fertilizer treatment, about 0.5 kg of Norwegian compound fertilizer (N15P15K15, Yara International, Oslo, Norway) was applied to each hole, dug at the center point between two trees. All treatments were carried out twice a year (in April and August). Soil samples were collected from all treatment plots one time per season (summer: June, autumn: September, winter: December, spring: March) in 2017 and 2018. Soil samples were collected at 0–10 cm depth in each plot using a five-point sampling method and then stored on dry ice and transported to a laboratory for analysis.
Soil microbial carbon and nitrogen analysis
The chloroform fumigation extraction method (Brookes et al. 1985) was used to analyze soil microbial biomass carbon (MBC) and nitrogen (MBN). A fresh soil sample of 20 g was fumigated with alcohol-free trichloromethane for 24 h, then extracted in 0.5 M K2SO4 (1:2.5 w/v). C and N contents were obtained using a TOC analyzer (multi N/C 3100, Analytik Jena, Germany).
Soil respiration measurement
Three polyvinyl chloride (PVC) collars (20 cm diameter and 10 cm height) were inserted 7 cm into the soil on the diagonal of each plot. Soil respiration rates were measured monthly from October 2017 to September 2018, using an LI-8100 automatic soil CO2 flux system (LI-COR, Lincoln, NE, USA). Measurements of soil respiration in all PVC collars were completed between 09:00 and 11:00 h on a sunny day. At the same time, soil temperature (T) and soil moisture (W) at 10-cm depth were measured by a TRIME-PICO TDR probe (IMKO, Ettlingen, Germany). The calculation to determine annual soil CO2 fluxes was described by Inoue and Koizumi (2012).
Soil nutrient, nitrogen mineralization and leaching determination
Soil samples were homogenized and fine roots, stones and other materials (> 2 mm) were discarded. Soil pH was measured using a glass electrode pH meter. Ammonium and nitrate nitrogen concentrations were measured using ion-selective electrodes (Greenberg et al. 1985). Available phosphorus was extracted using ammonium hydrochloride and determined using the molybdenum antimony colorimetric method (Olsen and Sommers 1982). Available potassium was determined using atomic absorption spectroscopy (Bahr et al. 2018).
Net N mineralization and leaching rates were measured in situ using a sequential coring technique (Adams and Attiwill 1986; Raison et al. 1987). Five sampling points were selected randomly along the diagonal line of each plot. At each sampling point, three PVC collars (4.6 cm in diameter and 15 cm in height) were hammered into the soil to a depth of 10 cm, and one of the three collars with soil (S0) was collected and taken to a laboratory for the various measurements of the soil. The other two collars were left in situ for 30 days. Another collar had an open top to allow rain to pass through (S1), and the other collar had a covered top and perforations in the upper 5 cm of the sidewall (S2) for ventilation. Net N mineralization was defined as the increase in ammonium plus nitrate N between (S0) and (S2), and net leaching was calculated between S0 and S1. Initial soil samples were collected in June (summer), September (autumn), December (winter) and March (spring) in the following year. Each soil sample was oven-dried, and the moisture content was measured by weighing method. A fresh soil sample of 10 g was mixed with 50 mL of 2 M KCl, shaken for 1 h, and filtered through filter paper. NH4-N and NO2-N + NO2-N concentrations were then measured using automated colorimetry.
Tree growth and haustorial number
Height and DBH of all trees in the 16 treatment plots were measured using a height meter and caliper, respectively, at the start of the experiment and 1 year later. Four 20 cm × 20 cm subplots were selected randomly in each plot within the vertical projection of the crown of S. album. The roots of all underground plant parts within each subplot were dug out, and the haustoria were counted.
Litter collection and analysis
Litter was collected monthly from October to September during 2017–2018. Three 1 m × 1 m litter traps 50 cm tall were placed across the diagonal of each plot. Litter from the three traps was mixed into one sample, oven-dried and weighed using an analytical scale (0.0001 g) (Souza et al. 2019). Total N, P and K of the litter were measured using the Kjeldahl method (Vanlauwe et al. 1996), phosphovanado-molybdate method of Hanson (1950) and flame photometry (Herrera et al. 2008), respectively.
Statistical analyses
A one-way ANOVA and least significant difference tests were used to determine the statistical significance of differences at the 0.05 level in mean soil microbial biomass, soil respiration, soil nutrient, nitrogen mineralization and leaching, litter production and growth increment in height and in DBH (Increment in height or DBH = Height or DBH 1 year after treatment − Height or DBH before treatment) in response to the weeding or fertilization treatments for each sampling date. All data were tested for homogeneity of variance and normality of residuals before conducting the ANOVA. Data analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Soil microbial biomass
Microbial biomass showed strongly seasonal variations with the highest in the summer and the lowest in the winter (Fig. 2). MBC and MBN contents differed depending on the treatment. In the spring after 1 year of treatment, MBC with weeding, fertilization and weeding + fertilization treatments decreased significantly by 34.1%, 27.8%, and 11.3%, respectively, compared with the control treatment (Fig. 2a). MBN in the weeding and fertilization treatments decreased significantly by 37.7% and 47.8% compared with the control (Fig. 2b). In general, all the treatments significantly reduced the soil microbial biomass compared to the controls.
Mean (± SD) soil microbial carbon (a, MBC) and nitrogen (b, MBN) in each season after different cultivation practices. Different letters above the histobars denote significant (p < 0.05) differences among treatments in the same season as determined by a one-way ANOVA and least significance difference test; blue lines represent standard deviations (n = 4). Control (CK: no weeding or fertilization), weeding (W), fertilization (F), and weeding + fertilization (W + F)
Soil respiration
Soil respiration rate fluctuated from month to month during the experiment; the unimodal curve for each treatment showed a maximum from May to November and minimum from December to April (Fig. 3a). CO2 flux values peaked in October and ranged from 7.28 to 10.48 μmol m−2 s−1. The CO2 flux ranged from 2.29 to 2.79 μmol m−2 s−1 and was lowest in February. During each month, the weeding + fertilization samples generally had the maximum soil respiration rate.
Monthly dynamics of soil respiration rate (a) and annual flux of soil respiration (b) under different treatments. Different letters denote significant (p < 0.05) differences among treatments in a one-way ANOVA and least significant difference test; bars represents standard deviations (n = 12). Control (CK, no weeding or fertilizer), weeding (W), fertilization (F)
Compared with the CK, the weeding, fertilization and weeding + fertilization treatments increased the annual flux in soil respiration by 22.2%, 17.1% and 45.5%, respectively (Fig. 3b). Thus, compared to CK, all the cultivation practices in this study significantly increased the soil respiration.
Soil nutrient, nitrogen mineralization and leaching
Soil pH (4.59–5.03) varied little among treatments throughout the year (Fig. 4a). The ammonium nitrogen content in the soil peaked in autumn for the three treatments and the CK (Fig. 4b). Compared with CK, ammonium nitrogen contents in the weeding and weeding + fertilization treatments increased significantly by 65.3 and 75.1%, respectively. Unlike ammonium nitrogen, nitrate nitrogen was highest in the spring (Fig. 4c). Nitrate nitrogen contents for all cultivation treatments were significantly greater than for the CK. Over the year, the nitrate nitrogen content for the CK was minimal compared to the three cultivation practices. Available phosphorus was highest in summer (Fig. 4d), and was significantly increased by weeding + fertilization over the four seasons. Available potassium for the three treatments was significantly greater than for the CK and was highest with weeding + fertilization (Fig. 4e). These results indicated that all the cultivation in this study significantly improved the nutrient levels of the plantation soil compared to the CK.
Soil pH (a), ammonium nitrogen (b), nitrate nitrogen (c), available phosphorus (d), available potassium (e) in different seasons after different treatments. Different letters denote significant (P < 0.05) differences among treatments in the same season as determined by a one-way ANOVA; error bars represent standard deviations (n = 4). Control (CK, no weeding or fertilizer), weeding (W), fertilization (F)
Nitrogen nitrification was higher in the spring and autumn, and nitrification rate was lowest in winter (Table 2). In addition, the nitrogen nitrification rate was lowest in the weeding treatment (1.39 mg kg−1 month−1). The CK group had the highest net nitrification rate in each season. The ammonium rate was highest in the fertilization treatment in the winter and lowest in the weeding treatment in the autumn. The net nitrogen ammonium rate had significant seasonal variation. Soil ammonia rates were lower in the autumn and spring. Ammonium nitrogen accumulated in the summer and winter and was highest in the winter (Table 2). Rates of nitrogen mineralization were higher in the spring and autumn. The leaching rate of soil nitrogen differed significantly among the seasons, and the mean leaching rate was highest in the autumn, followed by summer, spring and winter (Table 3). Briefly, the variation patterns in soil mineralization and nitrification rates were consistent with variations in the temperature throughout the year.
Compared with CK, the weeding + fertilization, fertilization, and weeding treatments significantly reduced annual mineralization by 7.5%, 20.3% and 22.9%, respectively (Fig. 5a). The annual nitrogen leaching in the weeding treatment was significantly lower than in the other treatments; the fertilization and weeding + fertilization treatments did not differ significantly from the CK (Fig. 5b). For all four treatments, the annual mineralization was greater than the annual nitrogen leaching. In brief, all cultivation practices significantly inhibited mineralization but did not increase leaching of soil nitrogen.
Tree growth
No statistically significant differences were found among the four treatments with regard to mean height and DBH for either species before treatments started (Table 4).
In the evaluation of increment change in height and DBH (Fig. 6), the greatest increase for D. odorifera (Fig. 6a) was found in the weeding + fertilization treatment (49.50 cm), followed by fertilization (42.40 cm), weeding (33.65 cm) and CK (27.90 cm). All cultivation treatments significantly increased the height increment compared to the CK. The DBH increment varied between 5.55 and 9.11 mm (Fig. 6c) and was significantly higher for the weeding + fertilization and fertilization treatments than in the CK; weeding did not significantly affect the DBH increment. Compared to the CK, all cultivation treatments increased the growth of D. odorifera.
Height increment for Dalbergia odorifera (a) and Santalum album (b) and DBH variation for D. odorifera (c) and S. album (d) under different treatments. Different letters denote significant (P < 0.05) differences among treatments in a one-way ANOVA and least significant difference test; bars represent standard deviations (n = ~ 120). Control (CK, no weeding or fertilizer), weeding (W), fertilization (F)
Unlike D. odorifera, S. album attained the greatest height increment in the fertilization treatment (Fig. 6b). Compared with the CK, the weeding and weeding + fertilization treatments led to significantly lower changes in height increment (30.03 and 23.6%, respectively). However, fertilization increased the height increment by 23.2%. The DBH increment with weeding was significantly lower than that in the CK, whereas fertilization and weeding + fertilization treatments did not differ significantly from the CK (Fig. 6d). Compared to the CK, fertilization significantly increased and weeding significantly reduced the growth of S. album.
Haustorium number
The number of S. album haustoria in plant roots after the various treatments is shown in Fig. 7. Significantly more haustoria formed in the fertilization treatment than in the other treatments, up to 2.94, 9.65, and 181.83 times as much as CK, weeding + fertilization, and only weeding, respectively. However, the haustorial number was much lower in the weeding and the weeding + fertilization treatments compared to CK. Thus, fertilization increased haustorial production, but weeding reduced haustorial production.
Number of Santalum album haustorium per square meter of soil in roots of Dalbergia odorifera under different treatments. Different letters denote a significant (p < 0.05) difference among treatments in a one-way ANOVA and least significant difference test; bars represent standard deviations (n = 16). Control (CK), weeding (W), fertilization (F)
Litter production
Total litter biomass differed significantly among treatments, ranging from 6.71 to 10.38 t ha−1 a−1 (Fig. 8A). Compared with levels in the CK, total litter biomass increased by 26.9% with fertilization, but decreased by 6.6% with weeding + fertilization and 18.0% with weeding.
Annual litter production (a) and nutrient content (b) under different treatments. Different letters denote significant (P < 0.05) differences among treatments in a one-way ANOVA and least significant difference test; bars represent standard deviation (n = 4). N: nitrogen; P: phosphorous; K: potassium. Control (CK), weeding (W), fertilization (F), and weeding + fertilization (W + F)
Annual total nutrient content of litter varied among treatments (Fig. 8b). On average, the annual litter nutrient content was in the order nitrogen (142.10−246.45 kg ha−1 a−1) > potassium (65.62–127.61 kg ha−1 a−1) > phosphorus (8.38–14.99 kg ha−1 a−1). The highest content of total litter nutrient was in the fertilization treatment (389.04 kg ha−1 a−1), whereas values in the fertilization and weeding + fertilization treatments did not differ significantly compared to the CK (Fig. 8b). Thus, compared to the CK, fertilization significantly increased the amount of litter and nutrient return, whereas weeding reduced the litter production of the mixed plantation.
Discussion
Effects of weeding and fertilization on soil biology process
In this mixed-species plantation, cultivation practices decreased microbial biomass, but increased soil respiration, indicating that both weeding and fertilization significantly affected soil biological processes. Soil microbial biomass has an important role in nutrient cycling and is therefore essential for plant growth, which is very sensitive to environmental factors (Fliessbach et al. 1994). Compared with CK, weeding and fertilization decreased soil microbial biomass in line with previous findings (Stewart et al. 2018; Sun et al. 2018). The effects of fertilization on soil communities depend heavily on the contents of soil nutrients (Allison and Martiny 2008). In N-limited soil, N addition will directly increase the microbial populations and activity (Hobbie and Vitousek 2000; Compton et al. 2004). On the contrary, N addition will decrease soil microbial biomass in the soils of N saturation and even N inhibition (Guo et al. 2017). Additionally, weeding reduced microbial biomass as a result of lower input of organic matter into the soil (Wardle et al. 1999).
Soil respiration rates in the different cultivation practices measured in this study were greater than in the CK, and weeding + fertilization generally yielded the highest rate. Total soil respiration consists of autotrophic and heterotrophic components (Wang et al. 2017). Autotrophic respiration is mainly from plant roots, and heterotrophic respirations is primarily from decomposition of organic matter by soil microbes (Zhao et al. 2018). Weeding and fertilization affect soil respiration through stimulating plant growth and altering microbial biomass and activity (Olsson et al. 2005; Allison and Martiny 2008). After our weeding treatments, the plant residues were spread evenly on the surface of the plantation soil, which allowed more organic matter to be returned to the soil and degraded. Because fertilization accelerates nutrient availability and improves root growth, the rate of root respiration increased in this study. These results are in line with those reported by Zhu et al. (2016), Nguyen and Marschner (2017) and Spohn and Schleuss (2019). Therefore, cultivation practices decreased soil microbial biomass, but increased soil respiration. They also promoted root growth and microbial activity and degradation ability, ultimately increasing root and microbial respiration.
Effects of weeding and fertilization on soil biochemical processes
In our study, all cultivation practices basically improved plantation nutrients. Soil nitrogen mineralization was inhibited, and leaching was not increased by the practices. Weeding and fertilization can improve sustainable and efficient use of nutrient of plantation land (Zhou et al. 2018). On the one hand, fertilization directly adds nutrients to the plantation soil (Bom et al. 2019), and plant residues left behind after weeding accelerate nutrient return to the soil (Tanaka et al. 2012). Consistent with the reduction in microbial populations by the cultivation practices in this study, the process of mineralization of soil nitrogen was inhibited, and leaching was not increased. Zhang et al. (2017) concluded that carbon/energy resources decrease with loss of plant diversity, thus leading to reduced soil microbial diversity. Therefore, in this study, weeding could inhibit soil nitrogen processes by reducing the microbial population. Furthermore, weeding + fertilization inhibited soil nitrogen processes less than weeding did because the combined practice added more nutrients required for microbial process.
The variations in soil mineralization and nitrification rates were consistent with the temperature variations throughout the year. Soil temperature is positively correlated with total nitrogen mineralization (Li et al. 2020). When the soil is full of water in the wet season, net nitrogen mineralization decreased. Therefore, soil mineralization rates were higher in the spring and autumn.
Overall, cultivation practices inhibited mineralization but did not increase nitrogen leaching from the soil, and the nutrient status in the plantation soil improved. The cultivation practices were thus beneficial for preserving soil fertility and accumulating nitrogen.
Effects of weeding and fertilization on tree growth of D. odorifera and S. album
For D. odorifera, all cultivation practices promoted higher growth compared to CK. Generally, weeding and fertilization can increase growth rates by controlling competing vegetation and increasing inputs to raise the availability of nutrients (Fox et al. 2007; Campoe et al. 2014). In many of these stands, fertilization will increase plant growth by increasing leaf area. For S. album, unlike for D. odorifera, growth was significantly increased by fertilization and decreased by weeding. These results are consistent with fertilization increasing and weeding decreasing the number of S. album haustoria in plant roots in this study. Although weeding removed nutrient-competing vegetation, it also reduced the nutrient sources in the host. Weeding promoted the growth of D. odorifera, while reducing the growth of S. album, perhaps due to the decrease in the number of haustoria that resulted in the weeding + fertilization and weeding treatments. Thus, the combined treatment of weeding and fertilization did not promote the expected higher growth compared to either of the single treatments or the lack of treatments (CK).
Inputs from litter, a major carbon and nutrient source, represent important components in the biogeochemistry in forest ecosystems (Attiwill et al. 1978). Changes in plant growth can also lead to the change in litter; the faster a plant grows, the more litter it produces (Belovsky and Slade 2000). In our study, fertilization significantly increased the amount of litter and nutrient return. The growth of trees increased rapidly with increased soil nutrients after fertilization as did the herbaceous shrubs; thus, S. album obtained more nutrients from these herbaceous hosts for its growth (Xu et al. 2011). Conversely, the weeding treatment significantly reduced the growth of S. album, resulting in decreased litter production.
In summary, the effects of the cultivation practices on the growth and litter production of D. odorifera and S. album were inconsistent. Fertilization significantly increased the growth of trees and the amount of litter and nutrient return, improving production in the mixed plantation of D. odorifera and S. album. However, weeding-related practices decreased the growth of trees and litter production in the mixed plantation by reducing the number of S. album haustorium in roots. Considering the short duration of the study, we need to continue monitoring tree growth for a few more years to determine whether these relationships continue over time.
Conclusion
In this mixed plantation, the effects of weeding and fertilization on soil biological processes are reflected in the reduction of microbial biomass and the increase of soil respiration. Weeding and fertilization inhibited mineralization but did not increase leaching of soil nitrogen, and nutrient status of the soil improved. Thus, these cultivation practices will help preserve soil fertility and the aid nitrogen accumulation. Inconsistent with non-parasitic plantations, the combined treatment of weeding and fertilization did not promote better growth compared to the single and control treatments. Cultivation practices improved the growth of D. odorifera, but weeding and weeding + fertilization decreased the growth of S. album and litter production in the mixed plantation because weeding decreased the number of S. album haustoria in roots. In conclusion, fertilization is recommended, but weeding practices are inappropriate for D. odorifera and S. album mixed plantations. These findings hold important implications for management practices for other parasite or mixed plantations.
References
Adams MA, Attiwill PM (1986) Nutrient cycling and nitrogen mineralization in eucalypt forests of south-eastern Australia. Plant Soil 92:341–362. https://doi.org/10.1007/BF02372483
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA 105:11512–11519. https://doi.org/10.1073/pnas.0801925105
Allison SD, Lu Y, Wei HC, Goulden ML, Martiny AC, Treseder KK, Martiny JB (2013) Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 94:714–725. https://doi.org/10.1890/12-1243.1
Attiwill PM, Guthrie HB, Leuning R (1978) Nutrient cycling in a Eucalyptus obliqua (L’Herit.) forest. I. Litter production and nutrient return. Aust J Bot 26:79–91. https://doi.org/10.1071/bt9780079
Bahr A, Ellström M, Bergh J, Wallander H (2018) Nitrogen leaching and ectomycorrhizal nitrogen retention capacity in a Norway spruce forest fertilized with nitrogen and phosphorus. Plant Soil 390:323–335. https://doi.org/10.1007/s11104-015-2408-6
Belovsky GE, Slade JB (2000) Insect herbivory accelerates nutrient cycling and increases plant production. Proc Natl Acad Sci USA 97:14412–14417. https://doi.org/10.1073/pnas.250483797
Bom F, Magid J, Jensen LS (2019) Long-term fertilization strategies and form affect nutrient budgets and soil test values, soil carbon retention and crop yield resilience. Plant Soil 434:47–64. https://doi.org/10.1007/s11104-018-3754-y
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard forest. For Ecol Manag 196:43–56. https://doi.org/10.1016/j.foreco.2004.03.011
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Campoe OC, Iannelli C, Stape JL, Cook RL, Mendes CJT, Viviane R (2014) Atlantic forest tree species responses to silvicultural practices in a degraded pasture restoration plantation: From leaf physiology to survival and initial growth. Forest Ecol Manag 313:233–242. https://doi.org/10.1016/j.foreco.2013.11.016
Carrero O, Stape JL, Allen L, Arrevillaga MC, Ladeira M (2018) Productivity gains from weed control and fertilization of short-rotation Eucalyptus plantations in the Venezuelan Western Llanos. Forest Ecol Manag 430:566–575. https://doi.org/10.1016/j.foreco.2018.07.050
Compton JE, Watrud LS, Porteous LA, DeGrood S (2004) Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard forest. Forest Ecol Manag 196:143–158. https://doi.org/10.1016/j.foreco.2004.03.017
Cui ZY, Yang ZJ, Xu DP, Xi RC, Zhang NN, Liu XJ, Hong Z (2017) Stem respiration and chemical composition in Dalbergia odorifera plantations differing in soil moisture content. Aust J For Sci 134:347–365. https://doi.org/10.3389/fpls.2019.00250
Cui ZY, Yang ZJ, Xu DP (2019) Synergistic roles of biphasic ethylene and hydrogen peroxide in wound-induced vessel occlusions and essential oil accumulation in Dalbergia odorifera. Front Plant Sci 10:250. https://doi.org/10.3389/fpls.2019.00250
Dhanya B, Syam V, Seema P (2010) Sandal (Santalum album L.) conservation in southern India: a review of policies and their impacts. J Trop Agric 48:1–10
Erb KH, Kastner T, Plutzar C, Bais ALS, Carvalhais N, Fetzel T, Gingrich S, Haberl H, Lauk C, Niedertscheider M, Pongratz J, Thurner M, Sebastiaan L (2018) Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 553:73–76. https://doi.org/10.1038/nature25138
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG (2012) Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci U S A 109:21390–21395. https://doi.org/10.1073/pnas.1215210110
Fliessbach A, Martens R, Reber HH (1994) Soil microbial biomass and microbial activity in soils treated with heavy metal contaminated sewage sludge. Soil Biol Biochem 26:1201–1205. https://doi.org/10.1016/0038-0717(94)90144-9
Fox TR (2000) Sustained productivity in intensively managed forest plantations. For Ecol Manage 138:187–202. https://doi.org/10.1016/S0378-1127(00)00396-0
Fox TR, Allen LH, Albaugh TJ, Rubilar R, Carlson CA (2007) Tree nutrition and forest fertilization of pine plantations in the southern United States. South J Appl For 31:5–11. https://doi.org/10.1093/sjaf/31.1.5
Greenberg BL (1985) Method and apparatus for the automatic identification and verification of commercial broadcast programs: U.S. Patent 4547804. 1985-10-15.
Guo P, Jia J, Han T, Xie JX, Wu PF, Du YH, Qu KY (2017) Nonlinear responses of forest soil microbial communities and activities after short-and long-term gradient nitrogen additions. Appl soil ecol 121:60–64. https://doi.org/10.1016/j.apsoil.2017.09.018
Hanson WC (1950) The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J Sci Food Agr 1:172–173. .https://doi.org/10.1002/jsfa.2740010604
Herrera F, Castillo JE, Chica AF, Bellido LL (2008) Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresource Technol 99:287–296. https://doi.org/10.1016/j.biortech.2006.12.042
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877. https://doi.org/10.1890/0012-9658(2000)081[1867:NLODIH]2.0.CO;2
Inoue T, Koizumi H (2012) Effects of environmental factors upon variation in soil respiration of a Zoysia japonica grassland, central Japan. Ecol Res 27:445–452. https://doi.org/10.1007/s11284-011-0918-0
Lee KH, Shibu J (2003) Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. For Ecol Manage 185:263–273. https://doi.org/10.1016/S0378-1127(03)00164-6
Li L, Zeng D, Yu Z, Fan Z, Mao R (2010) Soil microbial properties under N and P additions in a semi-arid, sandy grassland. Biol Fertil Soils 46:653–658. https://doi.org/10.1007/s00374-010-0463-y
Li Q, Zhou DW, Jin YH, Wang ML, Song YT, Li GD (2014) Effects of fencing on vegetation and soil restoration in a degraded alkaline grassland in northeast China. J Arid Land 6:478–487. https://doi.org/10.1007/s40333-013-0207-6
Li Q, Song X, Gu H, Gao F (2016) Nitrogen deposition and management practices increase soil microbial biomass carbon but decrease diversity in Moso bamboo plantations. Sci Rep-UK 6:28235. https://doi.org/10.1038/srep28235
Li Z, Zeng Z, Tian D, Wang JS, Fu Z, Wang BX, Tang Z, Chen W, Chen HY, Wang CH (2020) The stoichiometry of soil microbial biomass determines metabolic quotient of nitrogen mineralization. Environ Res Lett 15:034005. https://doi.org/10.1088/1748-9326/ab6a26
Lu JK, Xu DP, Kang LH, He XH (2014) Host-species-dependent physiological characteristics of hemiparasite Santalum album in association with N2-fixing and non-N2-fixing hosts native to southern China. Tree physiol 34:1006–1017. https://doi.org/10.1093/treephys/tpu073
Lu JK, Yang FC, Wang SK, Ma HB, Liang JF, Chen YL (2017) Co-existence of rhizobia and diverse non-rhizobial bacteria in the rhizosphere and Nodules of Dalbergia odorifera seedlings inoculated with Bradyrhizobium elkanii, rhizobium multihospitium-like and burkholderia pyrrocinia-like strains. Front microbiol 8:2255. https://doi.org/10.3389/fmicb.2017.02255
Nguyen TT, Marschner P (2017) Soil respiration, microbial biomass and nutrient availability in soil after addition of residues with adjusted N and P concentrations. Pedosphere 27:76–85. https://doi.org/10.1016/S1002-0160(17)60297-2
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. Am Soc Agron, Soil Sci Soc Am, Madison, Wisconsin, pp 403–430
Olsson P, Linder S, Giesler R, Hogberg P (2005) Fertilization of boreal forest reduces both autotrophic and heterotrophic soil respiration. Glob Change Biol 11:1745–1753. https://doi.org/10.1111/j.1365-2486.2005.001033.x
Özkan U, Gökbulak F (2017) Effect of vegetation change from forest to herbaceous vegetation cover on soil moisture and temperature regimes and soil water chemistry. Catena 149:158–166. https://doi.org/10.1016/j.catena.2016.09.017
Peng Y, Thomas SC, Tian D (2008) Forest management and soil respiration: implications for carbon sequestration. Environ Rev 16:93–111. https://doi.org/10.1139/A08-003
Pokharel P, Chang SX (2016) Exponential fertilization promotes seedling growth by increasing nitrogen retrains location in trembling aspen planted for oil sands reclamation. Forest Ecol Manag 372:35–43. https://doi.org/10.1016/j.foreco.2016.03.034
Pokharel P, Kwak JH, Chang SX (2017) Growth and nitrogen uptake of jack pine seedlings in response to exponential fertilization and weed control in reclaimed soil. Biol Fert Soils 53:701–713. https://doi.org/10.1007/s00374-017-1213-1
Raison RJ, Connell MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral-N in situ. Soil Biol Biochem 19:521–530. https://doi.org/10.1016/0038-0717(87)90094-0
Ramirez KS, Lauber CL, Knight R, Bradford MA, Fierer N (2010) Consistent effects of N fertilization on soil bacterial communities in contrasting systems. Ecology 91:3463–3470. https://doi.org/10.1890/10-0426.1
Rey A, Pegoraro E, Oyonarte C, Were A, Escribano P, Raimundo J (2011) Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biol Biochem 43:393–403. https://doi.org/10.1016/j.soilbio.2010.11.007
Samuelson L, Mathew R, Stokes T, Feng Y, Aubrey D, Coleman M (2009) Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For Ecol Manag 258:2431–2438. https://doi.org/10.1016/j.foreco.2009.08.020
Sida TS, Baudron F, Hadgu K, Derero A, Giller KE (2018) Crop vs tree: Can agronomic management reduce trade-offs in tree-crop interactions. Agr Ecosyst Environ 260:36–46. https://doi.org/10.1016/j.agee.2018.03.011
Song X, Peng C, Ciais P, Li Q, Xiang WH, Xiao WH, Zhou GM, Deng L (2020) Nitrogen addition increased CO2 uptake more than non-CO2 greenhouse gases emissions in a Moso bamboo forest. Sci Adv 6: eaaw5790. https://doi.org/10.1126/sciadv.aaw5790
Souza SR, Veloso MD, Espírito-Santo MM, Silva JO, Sánchez-Azofeifa A, Brito BGS, Fernandes GW (2019) Litterfall dynamics along a successional gradient in a Brazilian tropical dry forest. For Ecosyst 6:35. https://doi.org/10.1186/s40663-019-0194-y
Spohn M, Schleuss PM (2019) Addition of inorganic phosphorus to soil leads to desorption of organic compounds and thus to increased soil respiration. Soil Biol Biochem 130:220–226. https://doi.org/10.1016/j.soilbio.2018.12.018
Stewart CE, Roosendaal DL, Manter DK, Delgado JA, Del Grosso S (2018) Interactions of stover and nitrogen management on soil microbial community and labile carbon under irrigated no-till corn. Soil Sci Soc Am J 82:323–331. https://doi.org/10.2136/sssaj2017.07.0229
Sun DD, Xu QF, Tian T, Liu BR (2011) Investigation on soil microbial biomass and structure in Phyllostachys edulis plantations with increasing cultivation time. Sci Silva Sin 47:181–186
Sun H, Koal P, Gerl G, Schroll R, Gattinger A, Joergensen RG, Munch JC (2018) Microbial communities and residues in robinia-and poplar-based alley-cropping systems under organic and integrated management. Agroforestry syst 92:35–46. https://doi.org/10.1007/s10457-016-0009-x
Tanaka A, Toriyama K, Kobayashi K (2012) Nitrogen supply via internal nutrient cycling of residues and weeds in lowland rice farming. Field crop res 137:251–260. https://doi.org/10.1016/j.fcr.2012.09.005
Vanlauwe B, Nwoke OC, Sanginga N, Merckx R (1996) Impact of residue quality on the C and N mineralization of leaf and root residues of three agroforestry species. Plant Soil 183:221–231. https://doi.org/10.1007/BF00011437
Wang QK, Wang S, Huang Y (2008) Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For Ecol Manag 255:1210–1218. https://doi.org/10.1016/j.foreco.2007.10.026
Wang QK, Zhang WD, Sun T, Chen LC, Pang XY, Wang YP, Xiao FM (2017) N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric For Meteorol 232:66–73. https://doi.org/10.1016/j.agrformet.2016.08.007
Wardle DA, Yeates GW, Nicholson KS, Bonner KI, Watson RN (1999) Response of soil microbial biomass dynamics, activity and plant litter decomposition to agricultural intensification over a seven-year period. Soil Biol Biochem 31:1707–1720. https://doi.org/10.1016/S0038-0717(99)00090-5
Xu YR, Wang PC, Ji H, Dai GL, Li XS (2011) Influences of growth and arrangement distance of host plants on the growth of Santalum album artificial young plantation. Hubei Agricultural Sciences 50:4216–4220 (in Chinese)
Zhang XM, Johnston ER, Barberán A, Ren Y, Lü XT, Han XG (2017) Decreased plant productivity resulting from plant group removal experiment constrains soil microbial functional diversity. Global change biol 23:4318–4332. https://doi.org/10.1111/gcb.13783
Zhang Y, Wang LJ, Yuan YG, Xu J, Tu C, Fisk C, Zhang WJ, Chen X, Ritchie D, Hu SJ (2018) Irrigation and weed control alter soil microbiology and nutrient availability in North Carolina Sandhill peach orchards. Sci Total Environ 615:517–525. https://doi.org/10.1016/j.scitotenv.2017.09.265
Zhao B, Geng Y, Cao J, Yang L, Zhao XH (2018) Contrasting responses of soil respiration components in response to five-year nitrogen addition in a Pinus tabulaeformis forest in northern China. Forests 9:544. https://doi.org/10.3390/f9090544
Zhou XG, Zhu HG, Wen YG, Goodale UM, Li XQ, You YM, Liang HW (2018) Effects of understory management on trade-offs and synergies between biomass carbon stock, plant diversity and timber production in eucalyptus plantations. For Ecol Manag 410:164–173. https://doi.org/10.1016/j.foreco.2017.11.015
Zhu C, Ma YP, Wu HH, Sun T, Pierre KJ, Sun ZW, Yu Q (2016) Divergent effects of nitrogen addition on soil respiration in a semiarid grassland. Sci Rep-UK 6:33541. https://doi.org/10.1038/srep33541
Author information
Authors and Affiliations
Contributions
PZ wrote the manuscript. XFL and SYX collected data. ZJY and DPX developed the study design. ZYC provided expert knowledge used in the writing and revision of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study was supported by the National Key Research and Development Program (Grant No. 2016YFD0600205).
The online version is available at http:// www.springerlink.com
Corresponding editor: Zhu Hong
Peng Zhang and Xiaofei Li contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, P., Li, X., Xue, S. et al. Effects of weeding and fertilization on soil biology and biochemical processes and tree growth in a mixed stand of Dalbergia odorifera and Santalum album. J. For. Res. 32, 2633–2644 (2021). https://doi.org/10.1007/s11676-020-01286-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01286-5