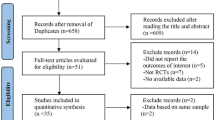
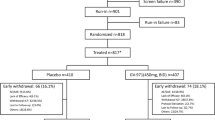
Abstract
In order to solve the problem of long-term (>9 months) efficacy in the treatment of Alzheimer’s disease (AD) by conventional therapy (CT), a staged and multiply-targeted sequential therapy based on the evolvement of patterns (STEP) was developed. Its main innovations include: (1) the time order of evolution of patterns defined by Chinese medicine (CM) in AD was found, that is, “the orderly pattern evolution starting from Shen (Kidney) deficiency, progressing to phlegm, stasis and fire, and worsening to severe toxin as well as functional collapse”; (2) the cascade hypothesis of Shen deficiency in AD and its sequential therapy based on Shen-reinforcing was proposed, that is, “reinforcing Shen in the early stage and throughout the whole process, resolving phlegm, activating blood and purging fire in the middle stage, detoxifying and replenishing vitality to stop the collapse in the advanced stage”, and through meta-analysis, clinical drug use was optimized, thus the leap from “inferential selection” to “evidence-based selection” was realized; (3) the STEP regimen combined with CT maintained cognitive and behavioral stability in AD patients for at least 12 months, with cognitive enhancement and behavioral synergy after 9 months, and cognitive benefit was superior to CT at 9, 12, 15, 18, 21, and 24 months, respectively. The 2-year cognitive improvement rate was increased by 25.64% (P=0.020) and the cognitive deterioration rate was decreased by 48.71% (P=0.000). Among them, the cognitive and functional benefits of Shen-reinforcing therapy for very early AD (350 cases) for 1 year were better than the placebo (P<0.001), and the dementia conversion rate was reduced by 8.85% (P=0.002). The behavioral symptomatic relief of patients with vascular dementia received fire-purging therapy (540 cases) was superior to those received CT (P=0.016). These data suggested that the STEP regimen has synergistic effects on CTs at least in terms of cognitive benefit, and the earlier the use, the greater the benefit will have. Therefore, the STEP regimen should be considered as one of the clinical options, particularly for the dearth of effective pharmaceutical or immunological interventions that are currently available for AD.
Similar content being viewed by others
References
Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet 2013;381:2016–2023.
Alzheimer’s Disease International. World Alzheimer Report 2015: The global impact of dementia. [2015–9–1] Available at https://doi.org/www.alz.co.uk/research/world-report-2015
Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid betaprotein. Ann N Y Acad Sci 2000;924:17–25.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–269.
Kua EH, Ho E, Tan HH, Tsoi C, Thng C, Mahendran R. The natural history of dementia. Psychogeriatrics 2014;14:196–201.
Winblad B, Wimo A, Engedal K, Soininen H, Verhey F, Waldemar G, et al. Three-year study of donepezil therapy in Alzheimer’s disease: effects of early and continuous therapy. Dement Geriatr Cogn Disord 2006;21:353–363.
Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 2007;22:806–812.
Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012;366:893–903.
Liu J, Wang LN, Tian J. Recognition of dementia in ancient China. Neurobiol Aging 2012;33:2948.
Tian J, Shi J, Zhang XK, Wang YY. Herbal therapy: a new pathway for the treatment of Alzheimer’s disease. Alzheimers Res Ther 2010;2:30.
Tian J, Shi, J, Miao Y, Mao M. A 24 week of randomized, double-blind, parallel controlled trial of GEPT extract in the treatment of amnestic mild cognitive impairment. Int J Neuropsychopharmacol 2010;131:140.
Tian J, Shi J, Zhang L, Yin J, Hu Q, Xu Y, et al. GEPT extract reduces A beta deposition by regulating the balance between production and degradation of A beta in APPV717I transgenic mice. Cur Alzheimer Res 2009;6:118–131.
Schneider LS, DeKosky ST, Farlow MR, Tariot PN, Hoerr R, Kieser M. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer’s type. Cur Alzheimer Res 2005;2:541–551.
Napryeyenko O, Sonnik G, Tartakovsky I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. J Neurol Sci 2009;283:224–229.
Herrschaft H, Nacu A, Likhachev S, Sholomov I, Hoerr R, Schlaefke S. Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J Psychiatr Res 2012;46:716–723.
Tian JZ. Case reports of brain diseases. Beijing: People’s Medical Publishing House;2015:99–100.
Shi J, Ni JN, Wei MQ, Zhang XK, Li T, Kang SH, Tian J. Association between pattern changes and cognitive outcome in Alzheimer’s disease. J Beijing Univ Tradit Chin Med (Chin) 2017;44:339–343.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 1991;12:383–388.
McDade E, Bateman RJ. Tau positron emission tomography in autosomal dominant Alzheimer’s disease: small windows, big picture. JAMA Neurol 2018;75:536–538.
Tian J, Shi J, Smallman R, Watsubo T, Mann DMA. Relationships in Alzheimer’s disease between the extent of Aβ deposition in cerebral blood vessel walls, as cerebral amyloid angiopathy, and the amount of cerebrovascular smooth muscle cells and collagen. Neuropathol Applied Neurobiol 2006;132:332–340.
Shi J, Tian J, Pritchard AH, Lendon C, Lambert JC, Iwatsubo T, et al. A 3′-UTR polymorphism in the oxidized LDL receptor 1 gene increases Aβ 40 load as cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol 2006;111:15–20.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016;537:50–56.
Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, et al. Efficacy and safety of tauaggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016;388:2873–2884.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of Solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018;378:321–330.
Pooler AM, Polydoro M, Maury EA, Nicholls SB, Reddy SM, Wegmann S, et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Commun 2015;3:14.
Gulisano W, Maugeri D, Baltrons MA, Fà M, Amato A, Palmeri A, et al. Role of amyloid-β and tau proteins in Alzheimer’s disease: confuting the amyloid cascade. J Alzheimers Dis 2018;64:S611–S631.
Sperling RA, Aisen PS, Laurel A, Beckettc LA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:280–292.
Long ZY, Shi J, Tian JZ, Wang YY. The study of symptom subtypes in dementia. Chin J Front Med Sci (Electronic Version, Chin) 2012;4:28–35.
Miao YC, Tian JZ, Shi J. Correlation between cognitive functions and syndromes of traditional Chinese medicine in amnestic mild cognitive impairment. J Chin Integr Med 2009;7:205–211.
Shi J, Tian J, Long Z, Liu X, Wei M, Ni J, et al. The pattern element scale: a brief tool of traditional medical subtyping for dementia. Evid Based Complement Alternat Med 2013;2013:460562.
Chen SH, Chen YL, Yang ZM. Initial study on disposition of Chinese medical symptoms and signs of mild cognitive impairment for elder people. World Chin Med (Chin) 2007;2:81–83.
Tian J. Dementia in an Asian Context. Johnson ML. The Cambridge handbook of age and aging. Cambridge: Cambridge University Press; 2005:261–273.
Tian JZ. Internal medicine of traditional Chinese medicine•dementia. Beijing: People’s Medical Publishing House;2012:122–128.
The Joint Consensus Group of Traditional Chinese Medicine on Alzheimer’s Disease. Consensus of traditional Chinese medicine specialists on Alzheimer’s disease. Chin J Integr Tradit West Med (Chin) 2018;38:523–529.
Tian JZ, Xie HG, Qin B, Fan DS, Gao J, Shi J. Chinese guidelines for the diagnosis and treatment of Alzheimer’s disease and other dementias. Beijing: People’s Medical Publishing House;2018:215–217.
Wang Y, Yang G, Tian J, Liu J. Ginseng for Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem 2016;16:529–536.
Shi J, Ni J, Lu T, Zhang XK, Wei MQ, Li T, et al. Adding Chinese herbal medicine to conventional therapy brings cognitive benefits to patients with Alzheimer’s disease: a retrospective analysis. BMC Complement Altern Med 2017;17:533–539.
Tian J, Shi J, Wei MQ, Qin RA, Chen YP, On behalf of the FFDS tablets clinical study investigators. Efficacy and safety of FFDS tablets in patients with mild to moderate vascular dementia: a 24-week randomized, double-blind, placebo, parallel-controlled trial. Alzheimer Dement 2013;9:S670–S671.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Nos. 81473518, 81573824, 81503625), Changjiang Scholar Program of Ministry of Education of the People’s Republic of China (No. IRT0810), Ministry of Education of the People’s Republic of China (No. B08006), Beijing Science and Technology Commission (No. Z141107002515019), and Beijing Health and Family Planning Commission (No. SF2014-1-4191)
Conflict of interest
The authors have no conflict of interests to declare.
Author Contributions
Tian JZ, Chen KJ and Wang YY are responsible for the overall framework design of this paper. Tian JZ, Shi J and Chen KJ are responsible for the design and evaluation of the clinical trials mentioned in the third part of this paper. Ni JN, Wei MQ and Zhang XK completed the systematic reviews, tables and figures in supplementary appendixes. Tian JZ and Zhang XK wrote the paper. Final manuscript was reviewed by all authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tian, Jz., Shi, J., Ni, Jn. et al. Sequential Therapy Based on Evolvement of Patterns: A New Model for Treatment of Alzheimer’s Disease. Chin. J. Integr. Med. 25, 565–573 (2019). https://doi.org/10.1007/s11655-019-3066-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-019-3066-y