Abstract
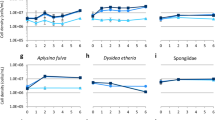
Sponges are among the most primitive multicellular organisms and well-known as a major source of marine natural products. Cultivation of sponge cells has long been an attractive topic due to the prominent evolutionary and cytological significance of sponges and as a potential approach to supply sponge-derived compounds. Sponge cell culture is carried out through culturing organized cell aggregates called ‘primmorphs.’ Most research culturing sponge cells has used unfractionated cells to develop primmorphs. In the current study, a tropical marine sponge Axinella sp., which contains the bioactive alkaloids, debromohymenialdisine (DBH), and hymenialdisine (HD), was used to obtain fractionated cells and the corresponding primmorphs. These alkaloids, DBH and HD, reportedly show pharmacological activities for treating osteoarthritis and Alzheimer’s disease. Three different cell fractions were obtained, including enriched spherulous cells, large mesohyl cells, and small epithelial cells. These cell fractions were cultivated separately, forming aggregates that later developed into different kinds of primmorphs. The three kinds of primmorphs obtained were compared as regards to appearance, morphogenesis, and cellular composition. Additionally, the amount of alkaloid in the primmorphs-culture system was examined over a 30-d culturing period. During the culturing of enriched spherulous cells and developed primmorphs, the total amount of alkaloid declined notably. In addition, the speculation of alkaloid secretion and some phenomena that occurred during cell culturing are discussed.







Similar content being viewed by others
References
Akpiri RU, Hodges NJ, Konya RS (2020) Aluminium induced DNA-damage and oxidative stress in cultures of the marine sponge Hymeniacidon perlevis. J Mar Sci. https://doi.org/10.30564/jms.v2i1.1070
Akpiri RU, Konya RS, Hodges NJ (2017) Development of cultures of the marine sponge Hymeniacidon perleve for genotoxicity assessment using the alkaline comet assay. Environ Toxicol Chem 36:3314–3323
Adamska M (2018) Differentiation and transdifferentiation of sponge cells. In Kloc M and Kubiak J (eds) Marine Organisms as Model Systems in Biology and Medicine. Springer, Cham, pp. 229–253. https://doi.org/10.1007/1978-1003-1319-92486-92481_92412
Annenkov VV, Danilovtseva EN (2016) Spiculogenesis in the siliceous sponge Lubomirskia baicalensis studied with fluorescent staining. J Struct Biol 194:29–37
Borisenko I, Podgornaya OI, Ereskovsky AV (2019) From traveler to homebody: Which signaling mechanisms sponge larvae use to become adult sponges? Adv Protein Chem Struct Biol. Elsevier:421–449
Cai X, Zhang Y (2014) Marine invertebrate cell culture: a decade of development. J Oceanogr 70:405–414
Cao X, Fu W, Yu X, Zhang W (2007) Dynamics of spicule production in the marine sponge Hymeniacidon perlevis during in vitro cell culture and seasonal development in the field. Cell Tissue Res 329:595–608.
Carballo JL, Yañez B, Zubía E, Ortega MJ, Vega C (2010) Culture of explants from the sponge Mycale cecilia to obtain bioactive mycalazal-type metabolites. Mar Biotechnol 12:516–525
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2020) Marine natural products. Nat Prod Rep 37:175–223
Chernogor L, Klimenko E, Khanaev I, Belikov S (2020) Microbiome analysis of healthy and diseased sponges Lubomirskia baicalensis by using cell cultures of primmorphs. PeerJ 8:e9080
Conkling M, Hesp K, Munroe S, Sandoval K, Martens DE, Sipkema D, Wijffels RH, Pomponi SA (2019) Breakthrough in Marine invertebrate cell culture: sponge cells divide rapidly in improved nutrient Medium. Sci Rep 9:1–10
Custodio MR, Prokic I, Steffen R, Koziol C, Borojevic R, Brümmer F, Nickel M, Müller WE (1998) Primmorphs generated from dissociated cells of the sponge Suberites domuncula: a model system for studies of cell proliferation and cell death. Mech Ageing Dev 105:45–59
Danilovtseva E, Pal'shin V, Zelinskiy S, Annenkov V (2019) Fluorescent dyes for the study of siliceous sponges. Limnology and Freshwater Biology:302–307
Eerkes-Medrano D, Feehan CJ, Leys SP (2015) Sponge cell aggregation: checkpoints in development indicate a high level of organismal complexity. Invertebr Biol 134:1–18
Ereskovsky AV (2010) The comparative embryology of sponges. Springer Science & Business Media;
Ereskovsky AV, Chernogor LI, Belikov SI (2016) Ultrastructural description of development and cell composition of primmorphs in the endemic Baikal sponge Lubomirskia baicalensis. Zoomorphology 135:1–17
Fuerst JA (2014) Diversity and biotechnological potential of microorganisms associated with marine sponges. Appl Microbiol Biotechnol 98:7331–7347
Funayama N (2018) The cellular and molecular bases of the sponge stem cell systems underlying reproduction, homeostasis and regeneration. Int J Dev Biol 62:513–525
Funayama N, Nakatsukasa M, Hayashi T, Agata K (2005a) Isolation of the choanocyte in the fresh water sponge, Ephydatia fluviatilis and its lineage marker, Ef annexin. Develop Growth Differ 47:243–253
Funayama N, Nakatsukasa M, Kuraku S, Takechi K, Dohi M, Iwabe N, Miyata T, Agata K (2005b) Isolation of Ef silicatein and Ef lectin as molecular markers sclerocytes and cells involved in innate immunity in the freshwater sponge Ephydatia fluviatilis. Zool Sci 22:1113–1122
Gökalp M, Wijgerde T, Sarà A, De Goeij JM, Osinga R (2019) Development of an integrated mariculture for the collagen-rich sponge Chondrosia reniformis. Mar Drugs 17:29. https://doi.org/10.3390/md17010029
Grasela JJ, Pomponi SA, Rinkevich B, Grima J (2012) Efforts to develop a cultured sponge cell line: revisiting an intractable problem. In Vitro Cellular & Developmental Biology-Animal 48:12–20
Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55:167–177
Huete-Stauffer C, Valisano L, Gaino E, Vezzulli L, Cerrano C (2015) Development of long-term primary cell aggregates from Mediterranean octocorals. In Vitro Cellular & Developmental Biology-Animal 51:815–826
Lanna E, Cajado B, Santos D, Cruz F, Oliveira F, Vasconcellos V (2018) Outlook on sponge reproduction science in the last ten years: are we far from where we should be? Invertebr Reprod Dev 62:133–142
Lavrov AI, Kosevich IA (2016) Sponge cell reaggregation: cellular structure and morphogenetic potencies of multicellular aggregates. J Exp Zool A Ecol Genet Physiol 325:158–177
Lavrov AI, Saidov DM, Bolshakov FV, Kosevich IA (2020) Intraspecific variability of cell reaggregation during reproduction cycle in sponges. Zoology:125795
Leal MC, Sheridan C, Osinga R, Dionísio G, Rocha RJM, Silva B, Rosa R, Calado R (2014) Marine microorganism-invertebrate assemblages: perspectives to solve the “supply problem” in the initial steps of drug discovery. Mar Drugs 12:3929–3952
Markl JS, Müller WE, Sereno D, Elkhooly TA, Kokkinopoulou M, Gardères J, Depoix F, Wiens M (2020) A synthetic biology approach for the fabrication of functional (fluorescent magnetic) bioorganic–inorganic hybrid materials in sponge primmorphs. Biotechnol Bioeng 117:1789–1804
Müller WE, Böhm M, Batel R, De Rosa S, Tommonaro G, Müller IM, Schröder HC (2000) Application of cell culture for the production of bioactive compounds from sponges: synthesis of avarol by primmorphs from Dysidea avara. J Nat Prod 63:1077–1081
Munroe S, Sandoval K, Martens DE, Sipkema D, Pomponi SA (2019) Genetic algorithm as an optimization tool for the development of sponge cell culture media. In Vitro Cellular & Developmental Biology-Animal 55:149–158
Musser JM, Schippers KJ, Nickel M, Mizzon G, Kohn AB, Pape C, Hammel JU, Wolf F, Liang C, Hernández-Plaza A (2019) Profiling cellular diversity in sponges informs animal cell type and nervous system evolution. BioRxiv:758276
Mussino F, Pozzolini M, Valisano L, Cerrano C, Benatti U, Giovine M (2013) Primmorphs cryopreservation: a new method for long-time storage of sponge cells. Mar Biotechnol 15:357–367
Padiglia A, Ledda FD, Padedda BM, Pronzato R, Manconi R (2018) Long-term experimental in situ farming of Crambe crambe (Demospongiae: Poecilosclerida). PeerJ 6:e4964
Peña JF, Alié A, Richter DJ, Wang L, Funayama N, Nichols SA (2016) Conserved expression of vertebrate microvillar gene homologs in choanocytes of freshwater sponges. EvoDevo 7:1–15
Pozzolini M, Mussino F, Cerrano C, Scarfì S, Giovine M (2014) Sponge cell cultivation: optimization of the model Petrosia ficiformis (Poiret 1789). J Exp Mar Biol Ecol 454:70–77
Qu Y, Song Y, Cao H, Zhang W (2012) Comparative study of morphological and molecular characters between two sponge specimens (Porifera: Demosponge: Axinella) with pharmacologically active compounds from the South China Sea. Pak J Zool 44:1727–1735
Rady H, Salem S, El-Arab ME (2019) Primmorph extracts and mesohyls of marine sponges inhibit proliferation and migration of hepatocellular carcinoma cells in vitro. Journal of pharmaceutical analysis 9:284–291
Rady HM, Hassan AZ, Salem SM, Mohamed TK, Esmaiel NN, Ez-El-Arab MA, Ibrahim MA, Fouda FK (2016) Induction of apoptosis and cell cycle arrest by Negombata magnifica sponge in hepatocellular carcinoma. Med Chem Res 25:456–465
Rocher C, Vernale A, Fierro-Constain L, Sejourne N, Chenesseau S, Marschal C, Le Golf E, Dutilleul M, Matthews C, Marschal F (2020) The buds of Oscarella lobularis (Porifera): a new convenient model for sponge cell and developmental biology. bioRxiv. https://doi.org/10.1101/2020.06.23.167296
Ruiz C, Valderrama K, Zea S, Castellanos L (2013) Mariculture and natural production of the antitumoural (+)-discodermolide by the Caribbean marine sponge Discodermia dissoluta. Mar Biotechnol 15:571–583
Schippers KJ, Martens DE, Pomponi SA, Wijffels RH (2011) Cell cycle analysis of primary sponge cell cultures. In Vitro Cellular & Developmental Biology-Animal 47:302–311
Simpson TL (2012) The cell biology of sponges. Springer-Verlag; New York
Singh A, Thakur NL (2015) Field and laboratory investigations of budding in the tetillid sponge Cinachyrella cavernosa. Invertebr Biol 134:19–30
Sipkema D, Osinga R, Schatton W, Mendola D, Tramper J, Wijffels RH (2005) Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis? Biotechnol Bioeng 90:201–222
Song Y-F, Qu Y, Cao X-P, Zhang W (2011) Cellular localization of debromohymenialdisine and hymenialdisine in the marine sponge Axinella sp. using a newly developed cell purification protocol. Mar Biotechnol 13:868–882
Sun L, Song Y, Qu Y, Yu X, Zhang W (2007) Purification and in vitro cultivation of archaeocytes (stem cells) of the marine sponge Hymeniacidon perleve (Demospongiae). Cell Tissue Res 328:223–237
Valisano L, Pozzolini M, Giovine M, Cerrano C (2012) Biosilica deposition in the marine sponge Petrosia ficiformis (Poiret, 1789): the model of primmorphs reveals time dependence of spiculogenesis. Ancient Animals, New Challenges. Springer, pp. 259–273
Vilanova E, Ciodaro PJ, Bezerra FF, Santos GR, Valle-Delgado JJ, Anselmetti D, Fernàndez-Busquets X, Mourão PA (2020) Adhesion of freshwater sponge cells mediated by carbohydrate–carbohydrate interactions requires low environmental calcium. Glycobiology 30:710–721
Vilanova E, Coutinho C, Maia G, Mourão PA (2010) Sulfated polysaccharides from marine sponges: conspicuous distribution among different cell types and involvement on formation of in vitro cell aggregates. Cell Tissue Res 340:523–531
Wilson H (1907) On some phenomena of coalescence and regeneration in sponges. J Elisha Mitchell Sci Soc 23:161–174
Zhang X, Cao X, Zhang W, Yu X, Jin M (2003) Primmorphs from archaeocytes-dominant cell population of the sponge Hymeniacidon perleve: improved cell proliferation and spiculogenesis. Biotechnol Bioeng 84:583–590
Funding
This research was funded by the China Scholarship Council (201908210162), the ‘Hi-Tech Research and Development Program of China’ (2006AA09Z435), and the National Natural Science Foundation of China (31801954).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Supplementary information
ESM 1
(DOCX 1.64 mb)
Rights and permissions
About this article
Cite this article
Song, Y., Qu, Y., Cao, X. et al. Cultivation of fractionated cells from a bioactive-alkaloid-bearing marine sponge Axinella sp.. In Vitro Cell.Dev.Biol.-Animal 57, 539–549 (2021). https://doi.org/10.1007/s11626-021-00578-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-021-00578-2