Abstract
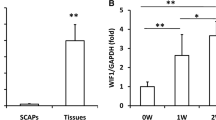
Attenuation of fibroblast growth factor receptor (FGFR) 2b signaling suppresses the differentiation of oral epithelial stem cells to ameloblasts, their survival and viability remaining unaffected; however, its effect on dentin formation is unknown. This study aimed to clarify the effect of attenuation of FGFR2b signaling on odontoblast differentiation and dentin formation. Initially, we used a murine rtTA transactivator/tetracycline promoter system for inducible and reversible attenuation of FGFR2b signaling in adult mice. Experimental animals overexpressed soluble FGFR2b (sFGFR2b), and wild-type controls were selected from the same litter (WT group). Histological analysis of CMV mice confirmed the obliteration of the enamel and ameloblast layer, and micro CT analysis revealed a significant increase in dentin thickness in CMV mice rather than in WT mice (P < 0.05). On analyzing the expression of dentin-related differentiation factors, DSPP, nestin, and OCN were upregulated in CMV mice compared to WT mice after 2 weeks of attenuation of FGFR2b signaling. Thereafter, on overexpressing sFGFR2b in dental pulp stem cells, RUNX2 and ALP were upregulated; however, DSPP, nestin, and OCN were downregulated in CMV mice compared to WT mice. The present results show that attenuation of FGFR2b signaling in the oral epithelium specifically induced odontoblast differentiation and promotes early-stage dentin calcification in dental pulp tissue.











Similar content being viewed by others
References
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P (1988) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A 95:5082–5087
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Brockes JP, Kumar A (2005) Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310:1919–1923
Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G (1998) Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17:1642–1655
D'Andrea LD, Del Gatto A, De Rosa L, Romanelli A, Pedone C (2009) Peptides targeting angiogenesis related growth factor receptors. Curr Pharm 15:2414–2429
De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C (2000) An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127:483–492
Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16:139–149
Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A 89:5547–5551
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Harada H, Nagai H, Mine N, Terada Y, Fujiwara H, Mikami I, Tsuneizumi M, Yabe A, Miyazaki K, Yokota T, Imoto I, Inazawa J, Emi M (2001) Molecular cloning, tissue expression, and chromosomal assignment of a novel gene encoding a subunit of the human signal-recognition particle. J Hum Genet 46:70–75
Itoh N, Ornitz DM (2008) Functional evolutionary history of the mouse fgf gene family. Dev Dyn 237:18–27
Itoh N, Ornitz DM (2011) Fibroblast growth factors from molecular evolution to roles in development, metabolism and disease. J Biochem 149:121–130
Kettunen P, Laurikkala J, Itäranta P, Vainio S, Itoh N, Thesleff I (2000) Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn 219:322–332
Kørbling M, Estrov Z (2003) Adult stem cells for tissue repair—a new therapeutic concept. N Engl J Med 349:570–582
Kuang-Hsien Hu J, Mushegyan V, Klein OD (2014) On the cutting edge of organ renewal: identification, regulation, and evolution of incisor stem cells. Genesis 52:79–92
Kurosaka H, Islam MN, Kuremoto K, Hayano S, Nakamura M, Kawanabe N, Yanagita T, Rice DP, Harada H, Taniuchi I, Yamashiro T (2011) Core binding factor beta functions in the maintenance of stem cells and orchestrates continuous proliferation and differentiation in mouse incisors. Stem Cells 29:1792–1803
Lan Y, Jia S, Jiang R (2014) Molecular patterning of the mammalian dentition. Semin Cell Dev Biol 25–26:61–70
Langer RS, Vacanti JP (1999) Tissue engineering: the challenges ahead. Sci Am 280:86–89
Mansukhani A, Bellosta P, Sahni M, Basilicoa C (2000) Signaling by fibroblast growth factors (fgf) and fibroblast growth factor receptor 2 (fgfr2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol 149:1297–1308
Nakamura T, Jimenez-Rojo L, Koyama E, Pacifici M, de Vega S, Iwamoto M, Fukumoto S, Unda F, Yamada Y (2017) Epiprofin regulates enamel formation and tooth morphogenesis by controlling epithelial-mesenchymal interactions during tooth development. J Bone Miner Res 32(3):601–610
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T (2007) The development of a bioengineered organ germ method. Nat Methods 4:227–230
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N (2000) FGF 10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277:643–649
Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M (1996) Receptor specificity of the fibroblast growth factor family. J Biol Chem 271:15292–15297
Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S, Saito M, Tsuji T (2011) Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One 6:e21531
Parsa S, Kuremoto K, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, Klein OD, Bellusci S (2010) Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development 137:3743–3752
Sagomonyants K, Kalajzic I, Maye P, Mina M (2015) Enhanced dentinogenesis of pulp progenitors by early exposure to FGF2. J Dent Res 94:1582–1590
Sauer B (1987) Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol 7:2087–2096
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21:138–141
Simann M, Le Blanc S, Schneider V, Zehe V, Lüdemann M, Schütze N, Jakob F, Schilling T (2017) Canonical FGFs prevent osteogenic lineage commitment and differentiation of human bone marrow stromal cells via ERK1/2 signaling. J Cell Biochem 118:263–275
Thesleff I, Hurmerinta K (1981) Tissue interactions in tooth development. Differentiation 18:75–88
Unda FJ, Martín A, Hilario E, Bègue-Kirn C, Ruch JV, Aréchaga J (2000) Dissection of the odontoblast differentiation process in vitro by a combination of FGF1, FGF2, and TGFbeta1. Dev Dyn 218:480–489
Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I (2007) An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol 5:e159
Watt FM, Hogan BL (2000) Out of Eden: stem cells and their niches. Science 287:1427–1430
Yamamoto N, Oshima M, Tanaka C, Ogawa M, Nakajima K, Ishida K, Moriyama K, Tsuji T (2015) Functional tooth restoration utilising split germs through re-regionalisation of the tooth-forming field. Sci Rep 5:18393
Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, Ohuchi H, Harada H (2006) Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development 133:1359–1366
Acknowledgments
Advice and comments given by Dr. Ichiro Takahashi, Department of Mucosal Immunology, Hiroshima University and Dr. Naoya Kakimoto, Department of Oral and Maxillofacial Radiology, Hiroshima University, have been a great help in this report. The authors thank Dr. Shinji Hiyama, Hiroshima University, and Dr. Shigeki Suzuki, Department of Periodontology and Endodontology, Tohoku University Graduate School of Dentistry, for providing technical support.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the National Institute of Biomedical Innovation in Japan (Project ID 16 K115920).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Hiroshima University Animal Experiment Facility (Hiroshima University Animal Experiment Approval No. A14-144, Genetically Modified Organism Experiment Approval No. 26-220).
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Yokoi, M., Kuremoto, Ki., Okada, S. et al. Effect of attenuation of fibroblast growth factor receptor 2b signaling on odontoblast differentiation and dentin formation. In Vitro Cell.Dev.Biol.-Animal 55, 211–219 (2019). https://doi.org/10.1007/s11626-019-00323-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00323-w