Abstract
Objectives
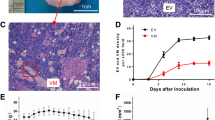
To evaluate quantitative three-dimensional (3D) dynamic contrast-enhanced ultrasound (DCE-US) in the assessment of tumor angiogenesis using an orthotropic liver tumor model.
Methods
Nine New Zealand white rabbits with liver orthotropic VX2 tumors were established and imaged by two-dimensional (2D) and 3D DCE-US after SonoVue® bolus injections. The intraclass correlation coefficients of perfusion parameters, including peak intensity (PI), mean transit time, time to peak, and area under the curve, were calculated based on time-intensity curve. The percentage area of microvascular (PAMV) and the expression of vascular endothelial growth factor (VEGF) were both evaluated by immunohistochemical analysis and weighted by the tumor activity area ratio. Correlations between quantitative and histologic parameters were analyzed.
Results
The reproducibility of 3D DCE-US quantitative parameters was excellent (ICC 0.91–0.99); but only PI showed high reproducibility (ICC 0.97) in 2D. None of the parameters of quantitative 2D DCE-US were significantly correlated with weighted PAMV or VEGF. For 3D DCE-US, there was a positive correlation between PI and weighted PAMV (r = 0.74, P = 0.04) as well as VEGF (r = 0.79, P = 0.02).
Conclusion
Quantitative parameters of 3D DCE-US show feasibility, higher reproducibility and accuracy for the assessment of tumor angiogenesis using an orthotropic liver tumor model compared with 2D DCE-US.




Similar content being viewed by others
Abbreviations
- au:
-
Arbitrary units
- AUC:
-
Area under the curve
- DCE-US:
-
Dynamic contrast-enhanced ultrasound
- ICC:
-
Intraclass correlation coefficient
- MTT:
-
Mean transit time
- PAMV:
-
Percentage area of microvascular
- PI:
-
Peak intensity
- ROI:
-
Region of interest
- 3D:
-
Three-dimensional
- TIC:
-
Time-intensity curve
- TTP:
-
Time to peak
- 2D:
-
Two-dimensional
- VEGF:
-
Vascular endothelial growth factor
- VOI:
-
Volume of interest
References
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6.
Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2000;87(12):881–6.
Marcus C, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72(3):217–38.
Greis C. Ultrasound contrast agents as markers of vascularity and microcirculation. Clin Hemorheol Microcirc. 2009;43(1–2):1–9.
Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc. 2011;49(1–4):137–49.
Bartolotta TV, Midiri M, Galia M, et al. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006;16(10):2234–41.
Ripolles T, Martinez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology. 2009;253(1):241–8.
Wang JW, Cao LH, Han F, et al. Contrast-enhanced US quantitatively detects changes of tumor perfusion in a murine breast cancer model during adriamycin chemotherapy. Acta Radiol. 2013;54(8):882–8.
Zhang HP, Shi QS, Li F, et al. Regions of interest and parameters for the quantitative analysis of contrast-enhanced ultrasound to evaluate the anti-angiogenic effects of bevacizumab. Mol Med Rep. 2013;8(1):154–60.
Zhou JH, Cao LH, Zheng W, Liu M, Han F, Li AH. Contrast-enhanced gray-scale ultrasound for quantitative evaluation of tumor response to chemotherapy: preliminary results with a mouse hepatoma model. Am J Roentgenol. 2011;196(1):W13–W1717.
Williams R, Hudson J, Lloyd B, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260(2):581–90.
Lassau N, Bonastre J, Kind M, et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors the french multicenter support for innovative and expensive techniques study. Investig Radiol. 2014;49(12):794–800.
Wang HJ, Lutz AM, Hristov D, Tian L, Willmann JK. Intra-animal comparison between three-dimensional molecularly targeted US and three-dimensional dynamic contrast-enhanced US for early antiangiogenic treatment assessment in colon cancer. Radiology. 2017;282(2):443–52.
Zhou J, Zhang H, Wang H, et al. Early prediction of tumor response to bevacizumab treatment in murine colon cancer models using three-dimensional dynamic contrast-enhanced ultrasound imaging. Angiogenesis. 2017;20(4):547–55.
El Kaffas A, Sigrist RMS, Fisher G, et al. Quantitative three-dimensional dynamic contrast-enhanced ultrasound imaging: first-in-human pilot study in patients with liver metastases. Theranostics. 2017;7(15):3745–58.
Wang HJ, Hristov D, Qin JL, Tian L, Willmann JK. Three-dimensional dynamic contrast-enhanced US imaging for early antiangiogenic treatment assessment in a mouse colon cancer model. Radiology. 2015;277(2):424–34.
Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology. 2004;233(1):191–9.
Lassau N, Koscielny S, Albiges L, et al. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16(4):1216–25.
Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification-preliminary results. Radiology. 2011;258(1):291–300.
Feingold S, Gessner R, Guracar IM, Dayton PA. Quantitative volumetric perfusion mapping of the microvasculature using contrast ultrasound. Investig Radiol. 2010;45(10):669–74.
Williams R, Hudson JM, Lloyd BA, et al. Dynamic microbubble contrast-enhanced us to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260(2):581–90.
Wang Z, Wang W, Liu GJ, et al. The role of quantitation of real-time 3-dimensional contrast-enhanced ultrasound in detecting microvascular invasion: an in vivo study. Abdom Radiol (N Y). 2016;41(10):1973–9.
Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37(1):25–35.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8.
Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64(9):2941–55.
Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993;10(4):302–13.
Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet (Lond Engl). 1992;340(8812):145–6.
Sharma S, Aggarwal N, Gupta S, Singh M, Gupta R, Dinda A. Angiogenesis in renal cell carcinoma: correlation of microvessel density and microvessel area with other prognostic factors. Int Urol Nephrol. 2011;43(1):125–9.
Shiyan L, Pintong H, Zongmin W, et al. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009;35(7):1086–91.
Mori N, Mugikura S, Takahashi S, et al. Quantitative analysis of contrast-enhanced ultrasound imaging in invasive breast cancer: a novel technique to obtain histopathologic information of microvessel density. Ultrasound Med Biol. 2017;43(3):607–14.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–80.
Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388(10043):518–29.
Wei X, Li Y, Zhang S, Ming G. Evaluation of thyroid cancer in Chinese females with breast cancer by vascular endothelial growth factor (VEGF), microvessel density, and contrast-enhanced ultrasound (CEUS). Tumour Biol. 2014;35(7):6521–9.
Lucidarme O, Kono Y, Corbeil J, et al. Angiogenesis: noninvasive quantitative assessment with contrast-enhanced functional US in murine model. Radiology. 2006;239(3):730–9.
Funding
Funding was provided by Natural Science Foundation of China (Grant no. 81601500), Natural Science Foundation of Guangdong Province (Grant nos. 2017A030313661, 2016A030310143), Medical Science and Technology Foundation of Guangdong Province (Grant no. 2017A020215195)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All animal experiments were complied with the ARRIVE guidelines and carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. All applicable institutional and national guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zheng, Q., Zhang, Jc., Wang, Z. et al. Assessment of angiogenesis in rabbit orthotropic liver tumors using three-dimensional dynamic contrast-enhanced ultrasound compared with two-dimensional DCE-US. Jpn J Radiol 37, 701–709 (2019). https://doi.org/10.1007/s11604-019-00861-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-019-00861-z