Summary
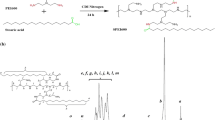
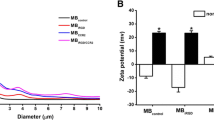
This study aimed to examine the preparation of cationic lipid microbubble (CLM), and evaluate its physical and chemical properties and toxicity, measure the gene transfection efficiency by ultrasound triggered microbobble destruction (UTMD) in combination with CLM. The CLM was prepared by the method of the thin film hydration, and its morphology was observed under the electron microscopy at 1st, 3rd, 7th, 10th, and 14th day after preparation, respectively. The size, Zeta potential and stability of CLM were tested. The acute toxicity of CLM was assessed. The green fluorescent protein gene (EGFP) transfection efficiency was evaluated. The experiment grouping was as follows: naked plasmid group (P group), ultrasonic irradiation plus naked plasmid group (P-US group), naked plasmid plus CLM group (P-CLM group), naked plasmid plus ultrasound and CLM group (UTMD group). The expression of EGFP was detected by fluorescent microscopy and flow cytometry. The results showed that CLMs were spherical in shape, with the similar size and good distribution degree under the light and electron microscopies. The size of CLMs was varied from 250.4±88.3 to 399.0±99.8 nm and the Zeta potential of CLMs from 18.80±4.97 to 20.1±3.1 mV. The EGFP expression was the strongest in the UTMD group, followed by the P-CLM group, P-US group and P group. Flow cytometry results were consistent with those of fluorescent microscopy. The transfection efficiency was substantially increased in the P-US group, P-CLM group and UTMD group as compared with that in the P group, almost 7 times, 10 times and 30 times higher than that in the P group respectively. It is suggested that CLMs prepared by the method of thin film hydration are uniform in diameter, and proved non-toxic. UTMD combined with CLM can significantly increase the transfection efficiency of EGFP to targeted cells.
Similar content being viewed by others
References
Ren J, Xu C, Zhou Z, et al. A novel ultrasound microbubble carrying gene and Tat peptide: preparation and characterization. Acad Radiol, 2009,16(12):1457–1465
Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol, 2006,41(3):354–362
Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res, 2009,42(7):881–892
Chomas JE, Dayton P, Allen J, et al. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control, 2001,48(1):232–248
Stride EP, Coussios CC. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H, 2010,224(2):171–191
Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev, 2008,60(10):1177–1192
Bekeredjian R, Grayburn PA, Shohet RV. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol, 2005,45(3): 329–335
Dijkmans PA, Juffermans LJ, Musters RJ, et al. Microbubbles and ultrasound: from diagnosis to therapy. Eur J Echocardiogr, 2004,5(4):245–256
Bouakaz A, Versluis M, de Jong N. High-speed optical observations of contrast agent destruction. Ultrasound Med Biol, 2005,31(3):391–399
de Jong N, Emmer M, van Wamel A, et al. Ultrasonic characterization of ultrasound contrast agents. Med Biol Eng Comput, 2009,47(8):861–873
Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun, 1991,179(1):280–285
Felgner JH, Kumar R, Sridhar CN, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem, 1994,269(4): 2550–2561
Le Gall T, Loizeau D, Picquet E, et al. A novel cationic lipophosphoramide with diunsaturated lipid chains: synthesis, physicochemical properties, and transfection activities. J Med Chem, 2010,53(4):1496–1508
Akowuah EF, Gray C, Lawrie A, et al. Ultrasound-mediated delivery of TIMP-3 plasmid DNA into saphenous vein leads to increased lumen size in a porcine interposition graft model. Gene Ther, 2005,12(4): 1154–1157
Bekeredjian R, Chen S, Frenkel PA, et al. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation, 2003,108(8):1022–1026
Chen S, Shohet RV, Bekeredjian R, et al. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol, 2003,42(2):301–308
Geis NA, Mayer CR, Kroll RD, et al. Spatial distribution of ultrasound targeted microbubble destruction increases cardiac transgene expression but not capillary permeability. Ultrasound Med Biol, 2009,35(7):1119–1126
Liang HD, Lu QL, Xue SA, et al. Optimisation of ultrasound-mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med Biol, 2004,30(11):1523–1529
Ohl CD, Arora M, Ikink R, et al. Sonoporation from jetting cavitation bubbles. Biophys J, 2006,91(11): 4285–4295
Song YK, Liu F, Chu S, et al. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum Gene Ther, 1997,8(3):1585–1594
Vitiello L, Chonn A, Wasserman JD, et al. Condensation of plasmid DNA with polylysine improves liposome-mediated gene transfer into established and primary muscle cells. Gene Ther, 1996,3(5):396–404
Smith DA, Porter TM, Martinez J, et al. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol, 2007,33(5):797–809
Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol, 2004,16(6–7):437–445
Unger EC, McCreery TP, Sweitzer RH, et al. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol, 1998,33(12):886–892
Chen L, ter Haar G, Hill CR, et al. Treatment of implanted liver tumors with focused ultrasound.Ultrasound Med Biol, 1998,24(9):1475–1488.
Liang HD, Lu QL, Xue SA, et al. Optimisation of ultrasound-mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med Biol, 2004,30(11):1523–1529
Fisher NG, Christiansen JP, Klibanov A, et al. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol, 2002,40(4):811–819
Janicki S, Jankowski J, Szulc J, et al. The effect of cryoprotectants on the physical properties of large liposomes containing sodium diclofenac. Acta Pol Pharm, 2002, 59(3):187–191
Hasik MJ, Kim DH, Howle LE, et al. Evaluation of synthetic phospholipid ultrasound contrast agents. Ultrasonics, 2002,40(9):973–982
El-Sherif DM, Lathia JD, Le NT, et al. Ultrasound degradation of novel polymer contrast agents. J Biomed Mater Res A, 2004,68(1):71–78
Caracciolo G, Callipo L, De Sanctis SC, et al. Surface adsorption of protein corona controls the cell internalization mechanism of DC-Chol-DOPE/DNA lipoplexes in serum. Biochim Biophys Acta, 2010,1798(3):536–543
Schratzberger P, Krainin JG, Schratzberger G, et al. Transcutaneous ultrasound augments naked DNA transfection of skeletal muscle. Mol Ther, 2002,6(5): 576–583
Leong-Poi H, Kuliszewski MA, Lekas M, et al. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res, 2007,101(3):295–303
Stride EP, Coussios CC. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H, 2010,224(2):171–191
Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc Dis, 2009, 27(Suppl 2):1–13
Ferrara KW, Borden MA, Zhang H. Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res, 2009,42(7):881–892
Tartis MS, Kruse DE, Zheng H, et al. Dynamic microPET imaging of ultrasound contrast agents and lipid delivery. J Control Release, 2008,131(3):160–166
Christiansen JP, French BA, Klibanov AL, et al. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol, 2003,29(12):1759–1767
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from National Natural Sciences Foundation of China (No. 81071280).
Rights and permissions
About this article
Cite this article
Zhang, L., Liu, Y., Xiang, G. et al. Ultrasound-triggered microbubble destruction in combination with cationic lipid microbubbles enhances gene delivery. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 31, 39–45 (2011). https://doi.org/10.1007/s11596-011-0147-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-011-0147-3