Abstract
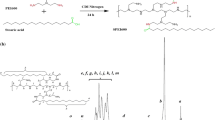
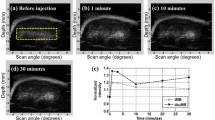
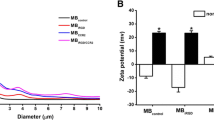
By adsorbing chitosan (CS)-functionalized Prussian blue (PB) nanoparticles (CS/PB NPs) complexing DNA onto the surface of gas encapsulated microbubbles (MBs), a multifunctional gene delivery system of MBs@CS/PB/DNA was fabricated for photothermally enhanced gene transfection through ultrasound-targeted microbubble destruction. CS/PB NPs of (2.69 ± 0.49) nm could complex DNA effectively when the mass ratio was 2:1. It was found that MBs@CS/PB/DNA could enhance ultrasound imaging greatly both in vitro and in vivo. In addition, MBs@CS/PB/DNA could be disrupted by applying a higher-intensity ultrasound irradiation to release CS/PB/DNA, which could effectively transform the near-infrared (NIR) light into heat to assist the uptake of CS/PB/DNA by cells. With the aid of ultrasound irradiation and NIR light irradiation, the gene transfection efficiency was significantly enhanced to (43.08 ± 1.13) %, much higher than polyethylenimine. Moreover, MBs@CS/PB/DNA showed excellent biocompatibility, encouraging the further exploration of MBs@CS/PB/DNA to be a platform for combined ultrasound image, photothermal therapy, drug delivery, and gene therapy.
摘要
使用壳聚糖修饰普鲁士蓝纳米粒子吸附DNA,再将其复合物吸附到超声微泡的表面,构成可以联合超声破碎和光热作用来共同提高基因转染效率的声光控基因转染平台。制备的壳聚糖修饰普鲁士蓝纳米粒子粒径约为2 nm,可以高效吸附DNA,其复合物吸附到超声微泡表面后构成了普鲁士蓝微泡。超声成像实验结果表明,该微泡可以显著提高超声成像效果。通过对普鲁士蓝微泡进行高强度超声辐照,可以破碎超声微泡,释放出壳聚糖修饰普鲁士蓝纳米粒子与DNA的复合物。再对该复合物进行近红外光照射,可以显著提高细胞对壳聚糖修饰普鲁士蓝纳米粒子与DNA复合物的摄取,从而提高基因转染效率。体外和体内实验验证了普鲁士蓝微泡具有很好的生物安全性,因此将普鲁士蓝微泡用于超声造影成像、光热治疗和基因治疗等方面都具有很好的应用前景。






Similar content being viewed by others
References
Wells DJ (2004) Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther 11:1363–1369
Yang ZR, Wang HF, Zhao J et al (2007) Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther 14:599–615
Niidome T, Huang L (2002) Gene therapy progress and prospects: nonviral vectors. Gene Ther 9:1647–1652
Matsushita-Ishiodori Y, Ohtsuki T (2012) Photoinduced RNA interference. Acc Chem Res 45:1039–1047
Feng LZ, Yang XZ, Shi XZ et al (2013) Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 9:1989–1997
Lu HD, Dai YH, Lv LL et al (2014) Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 9:e84703
Tang S, Huang ZX, Zhang HW et al (2014) Design and formulation of trimethylated chitosan-graft-poly(ε-caprolactone) nanoparticles used for gene delivery. Carbohydr Polym 101:104–112
Fu GL, Liu W, Feng SS et al (2012) Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem Commun 48:11567–11569
Li XD, Liang XL, Ma F et al (2014) Chitosan stabilized Prussian blue nanoparticles for photothermally enhanced gene delivery. Colloids Surf B Biointerfaces 123:629–638
Ferrara K, Pollard R, Borden M (2007) Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 9:415–447
Yang YB, Wang JR, Li XD et al (2015) A near infrared fluorescent/ultrasonic bimodal contrast agent for imaging guided pDNA delivery via ultrasound targeted microbubble destruction. RSC Adv 5:8404–8414
Zhang Q, Zhang L, Li J (2008) Fabrication and electrochemical study of monodisperse and size controlled Prussian blue nanoparticles protected by biocompatible polymer. Electrochim Acta 53:3050–3055
Basude R, Duckworth JW, Wheatley MA (2000) Influence of environmental conditions on a new surfactant-based contrast agent: ST68. Ultrasound Med Biol 26:621–628
Zhang J, Feng LZ, Tan XF et al (2013) Dual-polymer-functionalized nanoscale graphene oxide as a highly effective gene transfection agent for insect cells with cell-type-dependent cellular uptake mechanisms. Part Part Syst Charact 30:794–803
Liu RY, Luo HL, Feng HL et al (2002) A method to detect gene transfection efficacy by flow cytometry. Chin J Cancer 21:267–271
Li XD, Liang XL, Ma F et al (2014) Imaging guided photothermal therapy using iron oxide loaded poly(lactic acid) microcapsules coated with graphene oxide. J Mater Chem B 2:217–223
Jing LJ, Liang XL, Li XD et al (2013) Covalent attachment of Mn-porphyrin onto doxorubicin-loaded poly(lactic acid) nanoparticles for potential magnetic resonance imaging and pH-sensitive drug delivery. Acta Biomater 9:9434–9441
Liang XL, Li XD, Jing LJ et al (2013) Design and synthesis of lipidic organoalkoxysilanes for the self-assembly of liposomal nanohybrid cerasomes with controlled drug release properties. Chemistry 19:16113–16121
Liang XL, Li XD, Yue XL et al (2011) Conjugation of porphyrin to nanohybrid cerasomes for photodynamic diagnosis and therapy of cancer. Angew Chem Int Ed 50:11622–11627
White K, Rades T, Kearns P et al (2006) Immunogenicity of liposomes containing lipid core peptides and the adjuvant Quil A. Pharm Res 23:1473–1481
Zha ZB, Wang SM, Zhang SH et al (2013) Targeted delivery of CuS nanoparticles through ultrasound image-guided microbubble destruction for efficient photothermal therapy. Nanoscale 5:3216–3219
Ma M, Chen HR, Shi JL (2015) Construction of smart inorganic nanoparticle-based ultrasound contrast agents and their biomedical applications. Sci Bull 60:1170–1183
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81371580 and 21273014), the National Natural Science Foundation for Distinguished Young Scholars (81225011) and the State Key Program of National Natural Science of China (81230036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, X., Yue, X., Wang, J. et al. Prussian blue nanoparticle-loaded microbubbles for photothermally enhanced gene delivery through ultrasound-targeted microbubble destruction. Sci. Bull. 61, 148–156 (2016). https://doi.org/10.1007/s11434-015-0988-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-015-0988-4