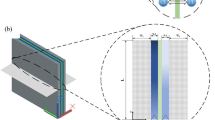
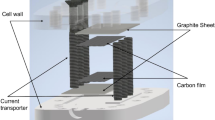
Abstract
With the development of the water treatment technology by capacitive deionization (CDI) method, some intercalation materials are gradually used in electrodes to enhance the desalination performance. In this paper, the mass conservation equation, energy conservation equation, and current conservation equation are coupled to establish a theoretical framework for modeling CDIs using intercalation materials, and the multi-physical fields such as the flow field, the electric field, and ion concentration distribution during the charging and discharging processes are analyzed. The effects of structural parameters such as the electrode thickness, spacer channel size, and ion exchange membrane thickness on the ion concentration distribution, intercalated-Na fraction distribution, volumetric energy consumption, salt adsorption capacity (SAC), and average salt adsorption rate (ASAR) are also investigated. The results show that during the charging process, the concentration increases from the inlet to the outlet on the left side of the membrane and decreases from the inlet to the outlet on the right side of the membrane. The intercalated-Na fraction distribution exhibits a certain degree of inhomogeneity in both the electrode thickness direction and the flow direction. An increased spacer channel size delays the time for the effluent concentration to reach its maximum, and increasing the thickness of the electrode not only has a similar effect, but also has a longer overall desalination time. Increasing the thickness of the ion exchange membrane significantly increases the magnitude of the cell voltage change, while the increase in electrode thickness significantly delays the time for the cell to reach the cutoff voltage. An increase in electrode thickness weakens the ASAR and increases the volumetric energy consumption, so choosing a smaller electrode thickness in the appropriate current density range can help improve the performance of the cell. Increasing the size of the spacer channel will weaken the concentration drop and SAC, so a larger spacer channel size is not conducive to the improvement of the desalination performance. Increasing the thickness of the ion exchange membrane leads to increased energy consumption and reduced SAC, so choosing a thinner ion exchange membrane thickness in the appropriate current density range can help improve the cell performance.








Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Khaled E, Mohammed K, Taha SE et al (2020) Environmental impact of desalination technologies: a review. Sci Total Environ 748:141528
Zhang P, Li J, Chan-Park MB (2020) Hierarchical porous carbon for high-performance capacitive desalination of brackish water. ACS Sustain Chem Eng 8(25):9291–9300
Wangwang T, Jie L, Di H et al (2019) Various cell architectures of capacitive deionization: recent advances and future trends. Water Res 150:225–251
Xudong Z, Kuichang Z, Xiaori Z et al (2020) Selective ion separation by capacitive deionization (CDI) based technologies: a state-of-the-art review. Environ Sci: Water Res Technol 6(2):243–257
Xiaoyu Z, Hongxin W, Huachao Z et al (2020) Electrode materials for capacitive deionization: a review. J Electroanal Chem 873:114416
Subramani A, Jacangelo JG (2015) Emerging desalination technologies for water treatment: a critical review. Water Res 75:164–187
Chen P-A, Cheng H-C, Wang HP (2018) Activated carbon recycled from bitter-tea and palm shell wastes for capacitive desalination of salt water. J Clean Prod 174:927–932
Xu X, Zhang S, Tang J et al (2020) Nitrogen-doped nanostructured carbons: a new material horizon for water desalination by capacitive deionization. Energy Chem 2(5):100043
Yang F, He Y, Rosentsvit L et al (2021) Flow-electrode capacitive deionization: a review and new perspectives. Water Res 200:117222
Zhang C, Ma J, Wu L et al (2021) Flow electrode capacitive deionization (FCDI): recent developments, environmental applications, and future perspectives. Environ Sci Technol 55(8):4243–4267
Liu Z, Shang X, Li H et al (2021) A brief review on high-performance capacitive deionization enabled by intercalation electrodes. Global Chall 5(1):2000054
Elisadiki J, King’ondu CK (2020) Performance of ion intercalation materials in capacitive deionization/electrochemical deionization: a review. J Electroanal Chem 878:114588
Folaranmi G, Bechelany M, Sistat P et al (2020) Towards electrochemical water desalination techniques: a review on capacitive deionization, membrane capacitive deionization and flow capacitive deionization. Membranes 10(5):96
Chen L, Xu X, Wan L et al (2021) Carbon-incorporated Fe3O4 nanoflakes: high-performance faradaic materials for hybrid capacitive deionization and supercapacitors. Mater Chem Front 5(8):3480–3488
Ding Z, Xu X, Li J et al (2022) Nanoarchitectonics from 2D to 3D: MXenes-derived nitrogen-doped 3D nanofibrous architecture for extraordinarily-fast capacitive deionization. Chem Eng J 430:133161
Singh K, Porada S, Gier HDD et al (2019) Timeline on the application of intercalation materials in capacitive deionization. Desalination 455:115–134
Li W, Dahn JR, Wainwright DS (1994) Rechargeable lithium batteries with aqueous electrolytes. Science 264(5162):1115–1118
Ramesh KG, Juan CI (2016) A brief review on multivalent intercalation batteries with aqueous electrolytes. Nanomaterials 6(3):41
Singh K, Bouwmeester HJM, Smet LCPMD et al (2018) Theory of water desalination with intercalation materials. Phys Rev Appl 9(6):064036
Lee J, Kim S, Kim C et al (2014) Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ Sci 7(11):3683–3689
Chen F, Huang Y, Guo L et al (2017) A dual-ion electrochemistry deionization system based on AgCl-Na 0.44 MnO 2 electrodes. Nanoscale 9(28):10101–10108
Seonghwan K, Jaehan L, Choonsoo K et al (2016) Na2FeP2O7 as a novel material for hybrid capacitive deionization. Electrochim Acta 203:265–271
Wang K, Liu Y, Ding Z et al (2021) Controlled synthesis of NaTi2(PO4)3/Carbon composite derived from metal-organic-frameworks as highly-efficient electrodes for hybrid capacitive deionization. Sep Purif Technol 278:119565
Chen Z, Xu X, Liu Y et al (2022) Ultra-durable and highly-efficient hybrid capacitive deionization by MXene confined MoS2 heterostructure. Desalination 528:115616
Chen Z, Ding Z, Chen Y et al (2023) Three-dimensional charge transfer pathway in close-packed nickel hexacyanoferrate−on−MXene nano-stacking for high-performance capacitive deionization. Chem Eng J 452:139451
Kevin H, Samuel W, Isaac C et al (2018) Prussian blue analogs as battery materials. Joule 2(10):1950–1960
Suss ME, Baumann TF, Bourcier WL et al (2012) Capacitive desalination with flow-through electrodes. Energy Environ Sci 5(11):9511–9519
Biesheuvel P (2009) Thermodynamic cycle analysis for capacitive deionization. J Colloid Interface Sci 332(1):258–264
Liu R, Yao S, Shen Y et al (2022) Numerical simulation of the water desalination process based on a modified Gouy-Chapman-Stern (GCS) model for a membrane capacitive deionization (MCDI) unit. Int J Electrochem Sci 17(220741):2
Wang L, Biesheuvel P, Lin S (2018) Reversible thermodynamic cycle analysis for capacitive deionization with modified Donnan model. J Colloid Interface Sci 512:522–528
Liu S, Smith KC (2019) Intercalated cation disorder in prussian blue analogues: first-principles and grand canonical analyses. J Phys Chem C 123(16):10191–10204
Erinmwingbovo C, Palagonia MS, Brogioli D et al (2017) Intercalation into a Prussian blue derivative from solutions containing two species of cations. Chem Phys Chem 18(8):917–925
Shrivastava A, Smith KC (2018) Electron conduction in nanoparticle agglomerates limits apparent Na + diffusion in Prussian blue analogue porous electrodes. J Electrochem Soc 165(9):A1777
Liu S, Smith KC (2018) Quantifying the trade-offs between energy consumption and salt removal rate in membrane-free cation intercalation desalination. Electrochim Acta 271:652–665
Smith KC (2017) Theoretical evaluation of electrochemical cell architectures using cation intercalation electrodes for desalination. Electrochim Acta 230:333–341
Porada S, Shrivastava A, Bukowska P et al (2017) Nickel hexacyanoferrate electrodes for continuous cation intercalation desalination of brackish water. Electrochim Acta 255:369–378
Smith KC, Dmello R (2016) Na-Ion Desalination (NID) Enabled by Na-blocking membranes and symmetric Na-intercalation: porous-electrode modeling. J Electrochem Soc 163(3):A530–A539
Shin Y-U, Lim J, Boo C et al (2021) Improving the feasibility and applicability of flow-electrode capacitive deionization (FCDI): Review of process optimization and energy efficiency. Desalination 502:114930
Zhao R, Satpradit O, Rijnaarts H et al (2013) Optimization of salt adsorption rate in membrane capacitive deionization. Water Res 47(5):1941–1952
Reale ER, Shrivastava A, Smith KC (2019) Effect of conductive additives on the transport properties of porous flow-through electrodes with insulative particles and their optimization for Faradaic deionization. Water Res 165:114995
Biesheuvel PM, Zhao R, Porada S et al (2011) Theory of membrane capacitive deionization including the effect of the electrode pore space. J Colloid Interface Sci 360(1):239–248
Liu R, Luo J, Yao S et al (2022) Three-dimensional lattice Boltzmann simulation of reactive transport and ion adsorption processes in battery electrodes of cation intercalation desalination cells. Sep Purif Technol 298:121626
Nordstrand J, Zuili L, Toledo-Carrillo EA et al (2022) Predicting capacitive deionization processes using an electrolytic-capacitor (ELC) model: 2D dynamics, leakages, and multi-ion solutions. Desalination 525:115493
Xiaobing W, Jinqiu L, Yang L et al (2021) Numerical analysis of capacitive deionization process using activated carbon electrodes. Water Air Soil Pollut 232(9):1–10
Amiri A, Vafai K (1998) Transient analysis of incompressible flow through a packed bed. Int J Heat Mass Transf 41(24):4259–4279
Shouguang Y, Zhangtian W, Rui L et al (2022) Influence of operation parameters and design parameters on desalination performance of Na-ion desalination battery. Ionics 28(4):1791–1807
Liu R, Yao S, Shen Y (2022) Pore-scale study of ion transport and intercalation processes of capacitive deionization cells with intercalation electrodes based on lattice Boltzmann method. Desalination 532:115718
Kamcev J, Paul DR, Manning GS et al (2018) Ion diffusion coefficients in ion exchange membranes: Significance of counterion condensation. Macromolecules 51(15):5519–5529
Shrivastava A, Do VQ, Smith KC (2022) Efficient, selective sodium and lithium removal by faradaic deionization using symmetric sodium titanium vanadium phosphate intercalation electrodes. ACS Appl Mater Interfaces 14(27):30672–30682
Ahn J, Lee J, Kim S et al (2020) High performance electrochemical saline water desalination using silver and silver-chloride electrodes. Desalination 476:114216
Taeyoung K, Jeyong Y (2015) CDI ragone plot as a functional tool to evaluate desalination performance in capacitive deionization. RSC Adv 5(2):1456–1461
Singh K, Zhang L, Zuilhof H et al (2020) Water desalination with nickel hexacyanoferrate electrodes in capacitive deionization: experiment, model and comparison with carbon. Desalination 496:114647
Funding
This work is financially supported by the National Natural Science Foundation of China (Grant Nos. 51906091 and 51776092) and Qinglan Project of Jiangsu Province of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Zhang, Q., Yao, S. et al. Numerical study of desalination characteristics of flow-by type cation intercalation desalination cells with different structural parameters. Ionics 29, 1431–1446 (2023). https://doi.org/10.1007/s11581-023-04907-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04907-1