Abstract
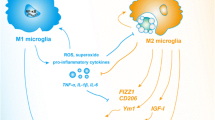
Neural inflammation immediately follows the onset of ischemic stroke. During this process, microglial cells can be activated into two different phenotypes: the M1 phenotype, which can worsen brain injury by producing pro-inflammatory cytokines; or the M2 phenotype, which can aid in long term recovery by producing anti-inflammatory cytokines. In this study, we formulate a nonlinear system of differential equations to model the activation of microglia post-ischemic stroke, which includes bidirectional switching between the microglia phenotypes, as well as the interactions between these cells and the cytokines that they produce. Further, we explore neuroprotectant-based modeling strategies to suppress the activation of the detrimental M1 phenotype, while promoting activation of the beneficial M2 phenotype. Through use of global sensitivity techniques, we analyze the effects of the model parameters on the ratio of M1 to M2 microglia and the total number of activated microglial cells in the system over time. Results demonstrate the significance of bidirectional microglia phenotype switching on the ratio of M1 to M2 microglia, in both the absence and presence of neuroprotectant terms. Simulations further suggest that early inhibition of M1 activation and support of M2 activation leads to a decreased minimum ratio of M1 to M2 microglia and allows for a larger number of M2 than M1 cells for a longer time period.





Similar content being viewed by others
References
American Stroke Association (2020) About stroke. https://www.stroke.org/en/about-stroke
Anderson W, Makadia H, Greenhalgh A, Schwaber J, David S, Vadigepalli R (2015) Computational modeling of cytokine signaling in microglia. Mol BioSyst 11(12):3332–3346
Boche D, Perry V, Nicoll J (2013) Activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39(1):3–18
Buzzard GT (2012) Global sensitivity analysis using sparse grid interpolation and polynomial chaos. Reliab Eng Syst Saf 107:82–89
Byrne J, Heidelberger R, Waxham M (2014) From molecules to networks: an introduction to cellular and molecular neuroscience. Academic Press, Cambridge
Centers for Disease Control and Prevention (2020) Stroke facts. https://www.cdc.gov/stroke/facts.htm
Cherry J, Olschowka J, O’Banion M (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm 11(1):98
Dronne M, Boissel J, Grenier E (2006) A mathematical model of ion movements in grey matter during a stroke. J Theor Biol 240(4):599–615
Dyker A, Lees K (1998) Duration of neuroprotective treatment for ischemic stroke. Stroke 29(2):535–542
Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, Begni B, Sarinella F, Frattola L, Simoni M (1999) Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab 19(9):1004–1009
Ginsberg M (2008) Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55(3):363–389
Gu B, Piebalgs A, Huang Y, Longstaff C, Hughes A, Chen R, Thom S, Xu X (2019) Mathematical modelling of intravenous thrombolysis in acute ischaemic stroke: effects of dose regimens on levels of fibrinolytic proteins and clot lysis time. Pharmaceutics 11(3):111
Guruswamy R, ElAli A (2017) Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Int J Mol Sci 18(3):496
Hao W, Friedman A (2016) Mathematical model on Alzheimer’s disease. BMC Syst Biol 10(1):108
Hu X, Li P, Guo Y, Wang H, Leak R, Chen S, Gao Y, Chen J (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070
Hu X, Leak R, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64
Jiang M, Liu X, Zhang D, Wang Y, Hu X, Xu F, Jin M, Cao F, Xu L (2018) Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J Neuroinflamm 15(1):78
Johnson W, Onuma O, Owolabi M, Sachdev S (2016) Stroke: a global response is needed. Bull World Health Organ 94(9):634-634A
Kent D, Selker H, Ruthazer R, Bluhmki E, Hacke W (2006) The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 37(12):2957–2962
Kleiner G, Marcuzzi A, Zanin V, Monasta L, Zauli G (2013) Cytokine levels in the serum of healthy subjects. Mediators Inflamm 2013
Ledeboer A, Brevé J, Poole S, Tilders F, Dam A (2000) Interleukin-10, interleukin-4, and transforming growth factor-\(\beta \) differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia 30(2):134–142
Lee Y, Lee S, Choi S, Yeo H, Chang K, Lee H (2014) Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. BioMed Res Int 2014
Lelekov-Boissard T, Chapuisat G, Boissel J, Grenier E, Dronne M (2009) Exploration of beneficial and deleterious effects of inflammation in stroke: dynamics of inflammation cells. Philos Trans Roy Soc A Math Phys Eng Sci 367(1908):4699–4716
Li C, Chen T, Zhou H, Feng Y, Hoi M, Ma D, Zhao C, Zheng Y, Lee S (2018) BHDPC is a novel neuroprotectant that provides anti-neuroinflammatory and neuroprotective effects by inactivating NF-\(\kappa \)B and activating PKA/CREB. Front Pharmacol 9:614
Li C, Bian Y, Feng Y, Tang F, Wang L, Hoi M, Ma D, Zhao C, Lee S (2019) Neuroprotective effects of BHDPC, a novel neuroprotectant, on experimental stroke by modulating microglia polarization. ACS Chem Neurosci 10(5):2434–2449
Li Y, He D, Zhang X, Liu Z, Zhang X, Dong L, Xing Y, Wang C, Qiao H, Zhu C, Chen Y (2012) Protective effect of celastrol in rat cerebral ischemia model: down-regulating p-JNK, p-c-Jun and NF-\(\kappa \)B. Brain Res 1464:8–13
Liu R, Liao X, Pan M, Tang J, Chen S, Zhang Y, Lu P, Lu L, Zou Y, Qin X, Bu L, Wan Q (2019) Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-\(\kappa \)B p65/Hif-1\(\alpha \) signaling pathway. J Immunol 202(6):1704–1714
Liu X, Liu J, Zhao S, Zhang H, Cai E, Cai M, Ji X, Leak R, Gao Y, Chen J, Hu X (2016) Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke 47(2):498–504
Liu X, Wen S, Yan F, Liu K, Liu L, Wang L, Zhao S, Ji X (2018) Salidroside provides neuroprotection by modulating microglial polarization after cerebral ischemia. J Neuroinflamm 15(1):1–11
Liu Z, Ran Y, Huang S, Wen S, Zhang W, Liu X, Ji Z, Geng X, Ji X, Du H, Leak R, Xiaoming H (2017) Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Front Aging Neurosci 9:233
Malek H, Ebadzadeh M, Safabakhsh R, Razavi A, Zaringhalam J (2015) Dynamics of the HPA axis and inflammatory cytokines: insights from mathematical modeling. Comput Biol Med 67:1–12
Minnerup J, Sutherland B, Buchan A, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13(9):11753–11772
Motto C, Ciccone A, Aritzu E, Boccardi E, Grandi C, Piana A, Candelise L (1999) Hemorrhage after an acute ischemic stroke. Stroke 30(4):761–764
Nakagawa Y, Chiba K (2014) Role of microglial M1/M2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals 7(12):1028–1048
Neuberger U, Kickingereder P, Schönenberger S, Schieber S, Ringleb P, Bendszus M, Pfaff J, Möhlenbruch M (2019) Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation ischemic stroke. Neuroradiology 61(4):461–469
Newton A, Lytton W (2016) Computer modeling of ischemic stroke. Drug Discov Today Dis Models 19:77–83
Olsen C, Ottesen J, Smith R, Olufsen M (2019) Parameter subset selection techniques for problems in mathematical biology. Biol Cybern 113(1–2):121–138
Orihuela R, McPherson C, Harry G (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173(4):649–665
Orlowski P, Chappell M, Park C, Grau V, Payne S (2011) Modelling of pH dynamics in brain cells after stroke. Interface Focus 1(3):408–416
Piebalgs A, Gu B, Roi D, Lobotesis K, Thom S, Xu X (2018) Computational simulations of thrombolytic therapy in acute ischaemic stroke. Sci Rep 8:15810
Qin C, Zhou L, Ma X, Hu Z, Yang S, Chen M, Bosco D, Wu L, Tian D (2019) Dual functions of microglia in ischemic stroke. Neurosci Bull 35:921–933
Rayz V, Boussel L, Lawton M, Acevedo-Bolton G, Ge L, Young W, Higashida R, Saloner D (2008) Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann Biomed Eng 36(11):1793–1804
Romero G, Martinez M, Félez J, Pearce G, Perkinson N (2011) Applicability of the GP device to the Circle of Willis arteries by using a mathematical model. In: 2011 UkSim 13th international conference on computer modelling and simulation. IEEE, pp 48–53
Russo C, Lagaert J, Chapuisat G, Dronne M (2010) A mathematical model of inflammation during ischemic stroke. In: ESAIM: proceedings, EDP Sciences, vol 30
Saltelli A, Tarantola S, Campolongo F, Ratto M (2004) Sensitivity analysis in practice: a guide to assessing scientific models. Wiley, Hoboken
Shao H, He Y, Li K, Zhou X (2013) A system mathematical model of a cell-cell communication network in amyotrophic lateral sclerosis. Mol BioSyst 9(3):398–406
Shoamanesh A, Kwok C, Lim P, Benavente O (2013) Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke 8(5):348–356
Singh P (2019) World stroke day 2019. https://www.who.int/southeastasia/news/speeches/detail/world-stroke-day-2019
Smith R (2013) Uncertainty quantification: theory, implementation, and applications. SIAM, Philadelphia
Taj S, Kho W, Aswendt M, Collmann F, Green C, Adamczak J, Tennstaedt A, Hoehn M (2016) Dynamic modulation of microglia/macrophage polarization by miR-124 after focal cerebral ischemia. J Neuroimmun Pharmacol 11(4):733–748
Tanaka T, Murakami K, Bando Y, Yoshida S (2015) Interferon regulatory factor 7 participates in the M1-like microglial polarization switch. Glia 63(4):595–610
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Taylor R, Sansing L (2013) Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol 2013
Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, Namas R, Constantine G, Zamora R, Puyana J, Vodovotz Y (2009) Mathematical modeling of posthemorrhage inflammation in mice: Studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock 32(2):172–178
Vaughan L, Ranganathan P, Kumar R, Wagner A, Rubin J (2018) A mathematical model of neuroinflammation in severe clinical traumatic brain injury. J Neuroinflamm 15(1):345
Wang Q, Tang X, Yenari M (2007) The inflammatory response in stroke. J Neuroimmunol 184(1–2):53–68
Wang Y, Yang T, Ma Y, Halade G, Zhang J, Lindsey M, Jin Y (2012) Mathematical modeling and stability analysis of macrophage activation in left ventricular remodeling post-myocardial infarction. BMC Genom 13:S21
Wentworth M, Smith R, Banks H (2016) Parameter selection and verification techniques based on global sensitivity analysis illustrated for an HIV model. SIAM/ASA J Uncertain Quant 4(1):266–297
Wu J, Dhingra R, Gambhir M, Remais J (2013) Sensitivity analysis of infectious disease models: methods, advances and their application. J R Soc Interface 10(86):20121018
Yenari M, Kauppinen T, Swanson R (2010) Microglial activation in stroke: therapeutic targets. Neurotherapeutics 7(4):378–391
Zhao S, Ma L, Chu Z, Xu H, Wu W, Liu F (2017) Regulation of microglial activation in stroke. Acta Pharmacol Sin 38(4):445–458
Acknowledgements
This work was partially supported by WPI’s Presidential Fellowship (S. Amato) and by the National Science Foundation under Grant Number NSF/DMS-1819203 (A. Arnold).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A Local Parameter and Initial Condition Ranges Maintaining Observed Trends Without Treatment
We perform a local, one-at-a-time analysis to determine individual ranges for the parameters and initial conditions over which Model (7) maintains the observed trends without terms simulating treatment. More specifically, we perturb each parameter individually while holding the others constant (fixed to their nominal values in Table 1). Table 3 lists the resulting ranges for each parameter over which it may be individually varied while maintaining the qualitative trends observed in the model output, assuming baseline initial conditions of \(M1(0)=100\) cells, \(M2(0)=100\) cells, \(P(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\), and \(A(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\). The qualitative trends maintained over these parameter ranges are a clear increase in pro-inflammatory cytokines after stroke onset, with anti-inflammatory cytokines remaining similar to starting level; early dominance of M2 microglia, with eventual takeover of M1 microglia; and increased levels of both M1 and M2 microglia over three days post-ischemic stroke.
Similarly, Table 4 gives ranges for each initial condition over which it may be individually varied while maintaining the qualitative trends observed in the model output, assuming that the model parameters are fixed to their nominal values in Table 1 and the remaining initial conditions are fixed to their baseline values. Further, Fig. 6 shows how the ratio of M1 to M2 microglia is affected when individually varying the initial conditions of Model (7) over larger ranges, while holding the others fixed at their baseline values. Note that while not much difference is observed beyond the first few hours when varying the initial values of M1 and M2, the initial levels of pro-inflammatory and anti-inflammatory cytokines have a more clear effect: When P(0) is large, as with \(P(0)=20 \ \frac{\mathrm{pg}}{\mathrm{ml}}\) in Fig. 6c, the ratio of M1 to M2 microglia does not go below 1, indicating there are always more detrimental M1 than beneficial M2 microglia in the system. Similar results hold when A(0) is too small, as seen in particular when \(A(0)=1 \ \frac{\mathrm{pg}}{\mathrm{ml}}\) in Fig. 6d.
Ratios of M1 to M2 microglial cells (M1 : M2) when varying each initial condition in Model (7) individually, while fixing the parameters to their nominal values in Table 1 and the remaining initial conditions to their baseline values of \(M1(0)=100\) cells, \(M2(0)=100\) cells, \(P(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\), and \(A(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\) (Color figure online)
Appendix B Effects of Microglia Phenotype Switching With and Without Treatment
The global sensitivity results presented in Figs. 3 and 5 highlight the significant influence of the parameters relating to bidirectional switching between microglia phenotypes on the corresponding model output, in particular when considering the ratio of M1 to M2 microglia. Here, we further analyze the effects of varying the values of these phenotype switching parameters (namely, \(s_{M1 \rightarrow M2}\) and \(s_{M2 \rightarrow M1}\)) on Model (7) and Model (21), respectively, thereby simulating situations both with and without treatment. Figure 7 shows how the ratio of M1 to M2 cells changes when varying the phenotype switching parameters between 0 and 1 for each model, highlighting values at which the behavior of the ratio changes.
In Fig. 7a, we see that when \(s_{M1 \rightarrow M2} = 0\) without treatment, there is an initial decrease of the ratio such that M2 is dominant for a short amount of time, after which the ratio grows in an exponential fashion. Similar behavior occurs for values of \(s_{M1 \rightarrow M2}\) between 0 and 0.05. When \(s_{M1 \rightarrow M2} = 0.05\) and up to 0.1, the ratio initially decreases and M2 dominates for a short amount of time, then begins to increase somewhat linearly. When \(s_{M1 \rightarrow M2} = 0.1\), the ratio shows an initial decrease below 1 followed by an increase and eventual leveling off at a value above 1. Similar behavior occurs for values of \(s_{M1 \rightarrow M2}\) between 0.1 and 0.25. When \(s_{M1 \rightarrow M2} =0.25\), the ratio begins under 1 and decreases slightly before increasing and leveling off at a value below 1. This behavior holds for values of \(s_{M1 \rightarrow M2}\) up to around 0.33. When \(s_{M1 \rightarrow M2} = 0.33\), the ratio begins under 1 and decreases slightly with no increase before leveling off at a value below 1. For values of \(s_{M1 \rightarrow M2}\) between 0.33 and 1, the ratio has an initial decrease and then gradually decreases for the full 72 h, with the ratio remaining below 1.
Figure 7b shows that when \(s_{M2 \rightarrow M1} =0\) without treatment, the ratio of M1 to M2 cells starts below 1 and decreases for the full time period, ending at a value below 1. This behavior occurs for values of \(s_{M2 \rightarrow M1}\) up until 0.12. At this value, the ratio has an initial decrease and then levels off around 15 h at a value below 1. For values of \(s_{M2 \rightarrow M1}\) between 0.12 and 0.16, the ratio decreases initially and then increases, ending at a value below 1 after 72 h. Between 0.16 and 0.6, the ratio has an initial decrease and then increases gradually, ending at a value above 1. For values of \(s_{M2 \rightarrow M1}\) larger than 0.6 and up until 0.8, the ratio begins at 1 and increases before moving to a more gradual linear-like increase. For \(s_{M2 \rightarrow M1}\) between 0.8 and 1, the ratio begins at 1 and increases in a linear manner, with a more significant increase over time.
In Fig. 7c, we observe that when \(s_{M1 \rightarrow M2} = 0\) with treatment applied, the ratio has a short decrease for about 1 h, followed by an increase that rises above 1 around 2.5 h. After this, there is another short decrease until around 10 h. The ratio then increases gradually until about the 45 h mark, at which point the increase becomes more significant. A similar behavior is observed for values of \(s_{M1 \rightarrow M2}\) up until 0.01. When \(s_{M1 \rightarrow M2} = 0.01\), we see a short initial decrease, followed by an increase; however, the ratio will not go above 1 in this increase. This is followed by a decrease until around 10 h. After this time, the ratio increases gradually and again begins to increase more significantly around 45 h. Similar behavior occurs for \(s_{M1 \rightarrow M2}\) values up until 0.05. At this value, we observe a short decrease (about 1 h), followed by a small increase with the ratio not going above 1. A decrease in the ratio occurs around 5 h and persists until about 10 h. After this, the ratio increases gradually until around the 45 h mark, where it then begins to increase with a steeper incline. This trend continues for values of \(s_{M1 \rightarrow M2}\) up until around 0.22. When \(s_{M1 \rightarrow M2} = 0.22,\) the ratio decreases gradually and levels off until around 45 h, after which it increases but ends at a value below 1. Similar behavior occurs for values of this parameter up until 0.5. When \(s_{M1 \rightarrow M2} = 0.5\), the ratio decreases until around the 45-h mark, at which point the ratio increases slightly until around 50 h where it then levels off at a value below 1. A similar trend occurs up until and at \(s_{M1 \rightarrow M2} = 1\).
Figure 7d shows that when \(s_{M2 \rightarrow M1}=0\) with treatment, the ratio of M1 to M2 cells begins below 1 and decreases until around 45 h. The ratio then increases slightly and levels off at a value under 1. Similar behavior occurs for values up until \(s_{M2 \rightarrow M1} = 0.1\). When \(s_{M2 \rightarrow M1} = 0.1\), the ratio decreases until around 45 h, after which it increases slightly for the rest of the 72 h and ends at a value under 1. This trend persists for values of \(s_{M2 \rightarrow M1}\) up until 0.2. When \(s_{M2 \rightarrow M1} = 0.2\), the ratio decreases gradually and then increases until around the 45-h mark, at which point it has a sharper increase and then increases more gradually, ending at a value above 1. This occurs for values up until \(s_{M2 \rightarrow M1} = 0.3\). When \(s_{M2 \rightarrow M1} = 0.3\) and up until 0.7, there is a short decrease in the ratio (for about 1 h), followed by an increase until around 4 h and another decrease until around 10 h. Then the ratio increases until the 45-h mark, at which point there is a sharper increase for the remaining time. When \(s_{M2 \rightarrow M1} = 0.7\), the ratio does not go below 1 for the full 72 h. Instead it increases gradually until around 45 h, when we see a steeper incline. A similar trend occurs for values up until \(s_{M2 \rightarrow M1} = 1\), with the ratio increasing more steeply at the end as \(s_{M2 \rightarrow M1}\) approaches 1.
Ratios of M1 to M2 microglial cells (M1 : M2) when varying each microglia phenotype switching parameter individually, while fixing the remaining model parameters to their nominal values in Table 1 and the initial conditions to their baseline values of \(M1(0)=100\) cells, \(M2(0)=100\) cells, \(P(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\), and \(A(0)=10 \ \frac{\mathrm{pg}}{\mathrm{ml}}\) (Color figure online)
Rights and permissions
About this article
Cite this article
Amato, S., Arnold, A. Modeling Microglia Activation and Inflammation-Based Neuroprotectant Strategies During Ischemic Stroke. Bull Math Biol 83, 72 (2021). https://doi.org/10.1007/s11538-021-00905-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-021-00905-4