Abstract
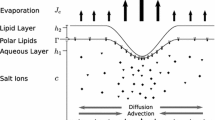
We present a mathematical model describing the spatial distribution of tear film osmolarity across the ocular surface of a human eye during one blink cycle, incorporating detailed fluid and solute dynamics. Based on the lubrication approximation, our model comprises three coupled equations tracking the depth of the aqueous layer of the tear film, the concentration of the polar lipid, and the concentration of physiological salts contained in the aqueous layer. Diffusive boundary layers in the salt concentration occur at the thinnest regions of the tear film, the black lines. Thus, despite large Peclet numbers, diffusion ameliorates osmolarity around the black lines, but nonetheless is insufficient to eliminate the build-up of solute in these regions. More generally, a heterogeneous distribution of solute concentration is predicted across the ocular surface, indicating that measurements of lower meniscus osmolarity are not globally representative, especially in the presence of dry eye.
Vertical saccadic eyelid motion can reduce osmolarity at the lower black line, raising the prospect that select eyeball motions more generally can assist in alleviating tear film hyperosmolarity. Finally, our results indicate that measured evaporative rates will induce excessive hyperosmolarity at the black lines, even for the healthy eye. This suggests that further evaporative retardation at the black lines, for instance due to the cellular glycocalyx at the ocular surface or increasing concentrations of mucus, will be important for controlling hyperosmolarity as the black line thins.








Similar content being viewed by others
Notes
That is, the volume of fluid evaporated per unit surface area per unit time.
References
Aydemir, E., Breward, C. J. W., & Witelski, T. P. (2011). The effect of polar lipids on tear film dynamics. Bull. Math. Biol., 73(6), 1171–1201.
Baudouin, C. (2007). The vicious circle in dry eye syndrome: a mechanistic approach. J. Fr. Ophthalmol., 30, 239–246.
Berger, R. E., & Corrsin, S. (1974). A surface tension gradient mechanism for driving the pre-corneal tear film after a blink. J. Biomech., 7, 225–238.
Berke, A., & Mueller, S. (1998). The kinetics of lid motion and its effects on the tear film. Adv. Exp. Med. Biol., 438, 417–424.
Braun, R. J. (2012). Dynamics of the tear film. Annu. Rev. Fluid Mech., 44, 267–297.
Braun, R. J., & Fitt, A. D. (2003). Modelling drainage of the precorneal tear film after a blink. Math. Med. Biol., 20, 1–28.
Bron, A., Tiffany, J., Gouveia, S., Yokoi, N., & Voon, L. (2004). Functional aspects of the tear film lipid layer. Exp. Eye Res., 78, 347–360.
Bron, A. J., Yokoi, N., Gaffney, E. A., & Tiffany, J. M. (2009). Predicted phenotypes of dry eye—proposed consequences of its natural history. Ocul. Surf., 7, 78–92.
Bron, A. J., Yokoi, N., Gaffney, E. A., & Tiffany, J. M. (2011a). A solute gradient in the tear meniscus I. A hypothesis to explain Marx’s line. Ocul. Surf., 9, 70–91.
Bron, A. J., Yokoi, N., Gaffney, E. A., & Tiffany, J. M. (2011b). A solute gradient in the tear meniscus II. Implications for lid margin disease, including meibomian gland dysfunction. Ocul. Surf., 9, 92–97.
Burdon, R. S. (1949). Surface tension and the spreading of liquids. London: Cambridge University Press.
Caffery, B. E., & Josephson, J. E. (1991). Corneal staining after sequential instillations of fluorescein over 30 days. Optom. Vis. Sci., 68, 467–469.
Craig, J., & Tomlinson, A. (1997). Importance of the lipid layer in human tear film stability and evaporation. Optom. Vis. Sci., 74, 8–13.
Deryagin, B. V., & Levi, S. M. (1964). Film coating theory. New York: Focal Point Press.
DEWS (2007). The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul. Surf., 5, 75–92.
Doane, M. G. (1980). Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am. J. Ophthalmol., 89, 507–516.
Doane, M. G. (1981). Blinking and the mechanics of the lacrimal drainage system. Ophthalmology, 88, 844–851.
Doughty, M. J., Naase, T., Donald, C., Hamilton, L., & Button, N. F. (2004). Visualisation of Marxs line along the marginal eyelid conjunctiva of human subjects with lissamine green dye. Ophthal. Physiol. Opt., 24, 1–7.
Gaffney, E. A., Tiffany, J. M., Yokoi, N., & Bron, A. J. (2010). A mass and solute balance model for tear volume and osmolarity in the normal and the dry eye. Prog. Retin. Eye Res., 29, 59–78.
Gilbard, J. P., Farris, R. L., & Santamaria, J. (1978). Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch. Ophthalmol., 96, 677–681.
Gilbard, J. P., Carter, J. B., Sang, D. N., Refojo, M. F., Hanninen, L. A., & Kenyon, K. R. (1984). Morphologic effect of hyperosmolarity on rabbit corneal epithelium. Ophthalmology, 91, 1205–1212.
Gilbard, J. P., Rossi, S. R., & Heyda, K. G. (1989). Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology, 96, 1180–1186.
Gipson, I. K. (2004). Distribution of mucins at the ocular surface. Exp. Eye Res., 78, 379–388.
Goto, E., Endo, K., Suzuki, A., Fujikura, Y., Matsumoto, Y., & Tsubota, K. (2003). Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci., 44, 533–539.
Harwood, M. R., Mezey, L. E., & Harris, C. M. (1999). The spectral main sequence of human saccades. J. Neurosci., 19, 9098–9106.
Heryudono, A., Braun, R. J., Driscoll, T. A., Maki, K. L., & Cook, L. P. (2007). Single-equation models for the tear film in a blink cycle: realistic lid motion. Math. Med. Biol., 24, 347–377.
Holly, F., & Lemp, M. A. (1973). Formation and rupture of the tear film. Exp. Eye Res., 15, 515–525.
Huang, A. J. W., Belldegrun, R., Hanninen, L., Kenyon, K. R., Tseng, S. C. G., & Refojo, M. F. (1989). Effect of hypertonic solutions on conjunctival epithelium and mucin like glycoprotein discharge. Cornea, 8, 15–20.
Johnson, M. E., & Murphy, P. J. (2005). The agreement and repeatability of tear meniscus height measurement. Methods Optom. Vis. Sci., 82, 1030–1037.
Jones, M. B., Please, C. P., McElwain, D. L. S., Fulford, G. R., & Robert, A. P. (2006). The effect of the lipid layer on tear film behaviour. Bull. Math. Biol., 86, 1355–1381.
Khanal, S., Tomlinson, A., McFadyen, A., Diaper, C., & Ramaesh, K. (2008). Dry eye diagnosis. Investig. Ophthalmol. Vis. Sci., 49, 1407–1414.
King-Smith, P. E., Fink, B. A., Hill, R. M., Koelling, K. W., & Tiffany, J. M. (2004). The thickness of the tear film. Curr. Eye Res., 29, 357–368.
Maki, K. L., Braun, R. J., Henshaw, W. D., & King-Smith, P. E. (2010a). Tear film dynamics on an eye-shaped domain I: pressure boundary conditions. IMA J. Math. Med. Biol., 27, 227–254.
Maki, K. L., Braun, R. J., Henshaw, W. D., & King-Smith, P. E. (2010b). Tear film dynamics on an eye-shaped domain part 2. Flux boundary conditions. J. Fluid Mech., 647, 361–390.
Martinez, C. C., Alanis, E. E., & Romero, G. G. (2002). Determinacion interferometrica del coeficiente de difusion de NaCl-H2O, a distintas concentraciones y temperaturas. Energ. Renov. Med. Ambiente, 10, 1–8.
Marx, E. (1924). Uber vitale farbung des auges und der augenlider. I. Uber anatomie, physiologie und pathologie des augenlidrandes und der tranenpunkte. Graf. Arch.Ophthalmol., 114, 465–482.
McCulley, J. P., & Shine, W. E. (2001). The lipid layer: the outer surface of the ocular surface tear film. Biosci. Rep., 21, 407–418.
McCulley, J. P., & Shine, W. E. (2002). Meibomian gland and tear film lipids: structure, function and control. Adv. Exp. Med. Biol., 506, 373–378.
Miller, K. L., Polse, K. A., & Radke, C. J. (2002). Black-line formation and the “perched” human tear film. Curr. Eye Res., 25, 155–162.
Mishima, S., & Maurice, D. M. (1961). The oily layer of the tear film and evaporation from the corneal surface. Exp. Eye Res., 1, 39–45.
Nagyova, A., & Tiffany, J. (1999). Components responsible for the surface tension of the human tears. Curr. Eye Res., 19, 4–11.
Nichols, B., Dawson, C. R., & Togni, B. (1983). Surface features of the conjunctiva and cornea. Investig. Ophthalmol. Vis. Sci., 24, 570–576.
Pult, H., Korb, D. R., Blackie, C., & Knop, E. (2010). About vital staining of the eye and eyelids. I. The anatomy, physiology, and pathology of the eyelid margins and the lacrimal puncta by E. Marx. Optom. Vis. Sci., 87, 718–724.
Riquelme, R., Lira, I., Pierez-Liopez, C. S., Rayas, J. A., & Rodriguez-Vera, R. (2007). Interferometric measurement of a diffusion coefficient: comparison of two methods and uncertainty analysis. J. Phys. D, Appl. Phys., 40, 2769–2776.
Sakata, E. K., & Berg, J. C. (1969). Surface diffusion in monolayers. Ind. Eng. Chem. Fundam., 8(3), 570–575.
Sharma, A., Tiwari, S., Khanna, R., & Tiffany, J. M. (1998). Hydrodynamics of meniscus induced thinning of the tear fluid. In D. Sullivan, D. Dartt, & M. Meneray (Eds.), Lacrimal gland, tear film, and dry eye syndromes 2, New York: Plenum.
Tabery, H. M. (2003). Corneal surface changes in keratoconjunctivitis sicca. Part I: the surface proper. A non-contact photomicrographic in vivo study in the human cornea. Eye, 17, 482–487.
Tiffany, J. M., Winter, N., & Bliss, G. (1989). Tear film stability and tear surface tension. Curr. Eye Res., 8, 507–515.
Tomlinson, A., & Khanal, S. (2005). Assessment of tear film dynamics quantification approach. Ocul. Surf., 3, 81–95.
Tsubota, K., Hata, S., Okusawa, Y., Egami, F., Ohtsuk, T., & Nakamori, K. (1996). Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch. Ophthalmol., 114, 715–720.
Winter, K. N., Anderson, D. M., & Braun, R. J. (2010). A model for wetting and evaporation of a post-blink precorneal tear film. Math. Med. Biol., 27, 211–225.
Wong, H., Fatt, I., & Radke, C. J. (1996). Deposition and thinning of the human tear film. J. Colloid Interface Sci., 184, 44–51.
Yarbus, A. L. (1967). Eye movements and vision. New York: Plenum.
Yokoi, N., Bron, A. J., Tiffany, J. M., Brown, N., Hsuan, J., & Fowler, C. (1999). Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Br. J. Ophthalmol., 83, 92–97.
Yokoi, N., Yameda, H., Mizukusa, Y., Bron, A. J., Tiffany, J. M., Kato, T., & Kinoshita, S. (2008). Rheology of tear film lipid layer spread in normal and aqueous tear-deficient dry eyes. Investig. Ophthalmol. Vis. Sci., 49, 5319–5324.
Zhu, H., & Chauhan, A. (2005). A mathematical model for ocular tear and solute balance. Curr. Eye Res., 30, 841–854.
Acknowledgements
This paper is based on work supported by Award No. KUK-C1-013-04 made by King Abdullah University of Science and Technology (KAUST). We are grateful to Professor Richard Braun, Professor Anthony Bron, Professor Colin Please, and Dr. John Tiffany for insightful discussions.
Author information
Authors and Affiliations
Corresponding author
Appendix: Derivation of the Lubrication Model
Appendix: Derivation of the Lubrication Model
We derive a model to describe the fluid motion, polar lipid concentration and salt concentration during a blink and during a saccade. In both cases, we neglect inertial terms. In addition, for saccadic motion we also neglect the non-inertial forces arising from the rotating frame, since it is straightforward to show that viscous effects dominate over rotational effects everywhere, even in the menisci. Hence, we proceed by substituting (29) and (30) into the model equations (3), (14), (17). Omitting the primes, we obtain the following system of equations:





where
and we note that we choose to retain u s as a primary variable. The boundary conditions (1), (2), (4)–(13), (15), (16), (18)–(24) become




where
The evaporation term in (57) is given by
The boundary conditions on the fluid problem are


where
and


The boundary conditions on the lipid problem are
while the boundary conditions on the salt problem are




The initial conditions are given by
where \(\tilde{h}_{0}(x)\) satisfies
Assuming that ε≪1, we can take advantage of the lubrication approximation. Following the usual routine of lubrication analysis, we solve the boundary-value problem for u, v and p expressing them as expansions in ε 2, which yields (e.g.)
Substituting (72) into (51), (57), and omitting O(ε 2) terms, we obtain
A similar system was previously derived by Jones et al. (2006) and Aydemir et al. (2011).
Let us now consider the equation for the salt concentration. Substituting the salt concentration expansion
into (52), (66), (67), and additionally assuming that Pe c ∼O(1) and \(\tilde{E}_{0}\sim O(1)\), our leading-order model is simply


Thus,
In order to find an equation for c 0, we proceed to next order in the field equations and boundary conditions, and find that



Integrating (78) across the tear film and using the boundary conditions (79), (80), we obtain
To obtain boundary conditions for Eqs. (73), (81), we use (65) and integrate (61), (68), (69) across the tear film, giving

Omitting O(ε 2) terms in Eqs. (71), we find that the initial tear film depth is given by
Rights and permissions
About this article
Cite this article
Zubkov, V.S., Breward, C.J.W. & Gaffney, E.A. Coupling Fluid and Solute Dynamics Within the Ocular Surface Tear Film: A Modelling Study of Black Line Osmolarity. Bull Math Biol 74, 2062–2093 (2012). https://doi.org/10.1007/s11538-012-9746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9746-9