Abstract
Background
In Europe, few data regarding the characteristics of EGFR exon 20 insertion (20ins) mutations in non-small cell lung cancer (NSCLC) are available.
Objective
Using a large real-world cohort, we assessed the incidence, characteristics, and outcomes of patients with non-squamous (nsq) NSCLC harboring EGFR exon 20ins.
Patients and Methods
The Epidemio-Strategy and Medical Economics advanced and metastatic lung cancer data platform including advanced/metastatic nsqNSCLC patients from January 2015 was analyzed (cut-off date: June 30, 2020). Characteristics, epidermal growth factor receptor (EGFR) mutation and other mutations, treatment patterns, and clinical outcomes were assessed for patients harboring EGFR exon 20ins, common EGFR mutations, other EGFR mutations, and wild-type EGFR. Survival parameters were estimated by the Kaplan-Meier method in these four groups.
Results
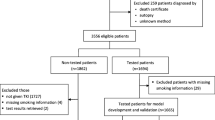
Out of 9435 nsqNSCLC patients tested for EGFR, 1549 (16.4%) had a mutation, including 61 with EGFR exon 20ins (3.9% of all mutated EGFR). These 61 patients had a mean age of 63.6 years, were mostly female (68.9%) and non-smokers (55.7%), with de novo stage IV disease (73.8%) and performance status 0–1 (76.9%). Almost all patients (95.1%) with exon 20ins received systemic therapy (median, three lines). First-line systemic treatments consisted mainly of combination chemotherapy (70.7%), single-agent EGFR tyrosine kinase inhibitors (10.3%), and single-agent immunotherapy (5.2%). After a median follow-up of 25.0 (95% confidence interval [CI] 22.3–32.4) months, the median real-world overall survival was 24.3 (19.1–32.6) months in patients with exon 20ins compared to 35.4 (95% CI 32.6–37.5) in patients with common EGFR mutation (n = 1049) (p = 0.049) and 19.6 (95% CI 18.6–20.5) in patients with wild-type EGFR (n = 7866) (p = 0.2).
Conclusions
This large national study in nsqNSCLC patients confirms that EGFR exon 20ins is a rare condition (0.6%). The prognosis associated with exon 20ins appears to be in line with that of wild-type EGFR, but worse than common EGFR mutations, highlighting the need for advancements for this rare population.



Similar content being viewed by others
References
Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:78985–93.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67.
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34.
Takeda M, Sakai K, Hayashi H, Tanaka K, Tanizaki J, Takahama T, et al. Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget. 2018;9:21132–40.
Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Reva BA, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12:220–9.
Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8:179–84.
Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–8.
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812–21.
Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther. 2016;9:4181–6.
Kate S, Chougule A, Joshi A, Noronha V, Patil V, Dusane R, et al. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non-small cell lung cancer patients: a single center retrospective analysis. Lung Cancer (Auckl). 2019;10:1–10.
Byeon S, Kim Y, Lim SW, Cho JH, Park S, Lee J, et al. Clinical Outcomes of EGFR exon 20 insertion mutations in advanced non-small cell lung cancer in Korea. Cancer Res Treat. 2019;51:623–31.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–237.
NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines Version 3.2020 Non-Small Cell Lung Cancer. 2020.
Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur J Cancer. 2008;44:946–53.
Burnett H, Emich H, Carroll C, Stapleton N, Mahadevia P, Li T. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: a systematic literature review. PLoS ONE. 2021;16:e0247620.
Choudhury NJ, Schoenfeld AJ, Flynn J, Falcon CJ, Rizvi H, Rudin CM, et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR exon 20 insertions. Clin Cancer Res. 2021;27:2920–7.
Xu CW, Wang WX, Wang D, Wang QM, Pu XX, Zhu YC, et al. Pemetrexed-based chemotherapy for non-small-cell lung cancer patients with EGFR exon 20 insertion mutation: a multicenter study. Transl Lung Cancer Res. 2020;9:1853–61.
Metro G, Baglivo S, Bellezza G, Mandarano M, Gili A, Marchetti G et al. Sensitivity to immune checkpoint blockade in advanced non-small cell lung cancer patients with EGFR exon 20 insertion mutations. Genes (Basel). 2021;12:679–690.
Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra77.
Yang GJ, Li J, Xu HY, Sun Y, Liu L, Li HS, et al. Osimertinib for Chinese advanced non-small cell lung cancer patients harboring diverse EGFR exon 20 insertion mutations. Lung Cancer. 2021;152:39–48.
Pacini L, Jenks AD, Vyse S, Wilding CP, Arthur A, Huang PH. Tackling drug resistance in EGFR exon 20 insertion mutant lung cancer. Pharmgenomics Pers Med. 2021;14:301–17.
Riely GJ, Neal JW, Camidge DR, Spira AI, Piotrowska Z, Costa DB, et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase 1/2 trial. Cancer Discov. 2021;11(7):1688–99.
Horn L, Lin HM, Padda SK, Aggarwal C, McCoach CE, Zhu Y, et al. Indirect comparison of TAK-788 vs real-world data outcomes in refractory non-small cell lung cancer (NSCLC) with EGFR exon 20 insertions. J Clin Oncol. 2020;38(15_suppl):9580.
Park K, John T, Kim S-W, Lee JS, Shu CA, Kim D-W, et al. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15_suppl):9512.
Yun J, Lee SH, Kim SY, Jeong SY, Kim JH, Pyo KH, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR Exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10:1194–1209.
Acknowledgements
We thank the 30 health facilities involved in the ESME ALMC database as of June 30, 2020 for providing the data and each ESME local coordinator for managing the project at local level: Centre François Baclesse, Caen; Institut de Cancérologie de l’Ouest, Angers/Nantes; Centre Leon Bérard, Lyon, Institu Curie, Paris/ Saint-Cloud; Centre Antoine Lacassagne, Nice; Centre Jean Perrin, Clermont Ferrand; Centre Oscar Lambret, Lille; Institut Claude Regaud, Toulouse; Centre Hospitalier Régional Universitaire, Tours; Centre Georges-François Leclerc, Dijon; Institut Paoli-Calmettes, Marseille; Centre Paul Strauss, Strasbourg; Institut de Cancérologie de Lorraine, Nancy; Gustave Roussy, Villejuif; Centre Hospitalier Intercommunal, Créteil; Centre Hospitalier, Mulhouse; Institut Bergonié, Bordeaux; Institut Jean Godinot, Reims; Centre Hospitalier Métropole Savoie, Chambery; Centre Henri Becquerel, Rouen; Centre Hospitalier, Côte Basque, Bayonne; Centre Hospitalier Universitaire, Rennes; Centre Hospitalier Sainte-Musse, Toulon; Centre Hospitalier, Départemental de Vendée, La Roche-sur-Yon; Institut de Cancerologie Mutualiste, Montpellier; HEM, Marseille; Centre Hospitalier, Annecy-Genevois, Annecy; Centre Hospitalier, Saint-Quentin; Centre Hospitalier Universitaire, d’Angers. Moreover, we thank the ESME Scientific Committee members for their ongoing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The ESME AMLC database receives financial support from an industrial consortium (AstraZeneca, MSD, BMS, Janssen, Amgen, and Roche). Data collection, analysis, and publication are fully managed by Unicancer independently of the industrial consortium.
Conflicts of interest
CC: Grants, personal fees, and non-financial support from AstraZeneca, Boehringer Ingelheim, GSK, Roche, Sanofi, BMS, MSD, Lilly, Novartis, Takeda, Bayer, and Amgen. TF received fees from Cellectis that were compensated by his institution. DD: No conflict of interest. MP: Grants, personal fees, and non-financial support from AstraZeneca, Roche, Boehringer Ingelheim, and Takeda, outside the submitted work. NG: Research/grant support from MS, AstraZeneca, AbbVie, Amgen, Boehringer-Ingelheim, Eli Lilly, Hoffmann-La Roche, Janssen, Merck, MSD, Novartis, Pfizer, Sivan, and Trizell and consultative services for BMS, AstraZeneca, AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Hoffmann-La Roche, Janssen, Merck, MSD, Novartis, Pfizer, Sanofi, and Sivan. ED: No conflict of interest. HL: Consulting fees from BMS, AstraZeneca, Takeda, Roche, Pfizer, Novartis, and Lilly. JO: No conflict of interest. DP: Consulting, advisory role or lectures for AstraZeneca, AbbVie, Bristol-Myers Squibb, Celgene, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, Roche, Samsung, and Janssen; travel, accommodation, and expenses for meetings for AstraZeneca, Roche, Novartis, prIME Oncology, and Pfizer; and advisory role for AstraZeneca, AbbVie, Bristol-Myers Squibb, Celgene, Merck, Novartis, Pfizer, Roche, Samsung, and Janssen. CK: No conflict of interest. SH: Fees from Takeda and AstraZeneca to participate in review activities. EP: Consulting fees from Roche, Astra Zeneca, BMS, MSD, and Takeda and support for meetings from Takeda and MSD. CCD: No conflict of interest. GC: No conflict of interest. GS: No conflict of interest. LB: No conflict of interest. XQ: No conflict of interest. RG, SC, RS, AM, and PDL did not report conflicts of interest.
Ethics approval
Unicancer manages the ESME AMLC data platform in accordance with current best practice guidelines. In compliance with French regulations, the ESME data platform was authorized by the French data protection authority (initial authorization no. DE-2017-397 and subsequent amendment dated October 14, 2019 in accordance with General Data Protection Regulation). Data are updated on a yearly basis and run into a data-management process aimed at ensuring the quality of the data analyzed.
Informed consent
Not applicable.
Data availability
Individual participant data that underlie the reported results will not be made available.
Authors’ contributions
Conceptualization: CC, TF, DD, and XQ. Formal analysis: GC. Methodology: CC, TF, DD, GS, LB, and XQ. Project administration: GS and LB. Resources: GS and LB. Software: GC. Supervision: CC, TF, DD, and XQ. Validation: GS and LB. Visualization: GC. Writing—original draft: CC and LB. Writing—review and editing: TF, DD, MP, NG, ED, HL, RG, SC, JO, RS, DP, AM, CK, PDL, SH, EP, CCD, LB, and XQ.
Rights and permissions
About this article
Cite this article
Chouaid, C., Filleron, T., Debieuvre, D. et al. A Real-World Study of Patients with Advanced Non-squamous Non-small Cell Lung Cancer with EGFR Exon 20 Insertion: Clinical Characteristics and Outcomes. Targ Oncol 16, 801–811 (2021). https://doi.org/10.1007/s11523-021-00848-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00848-9