Abstract
Background
EGFR mutated lung cancer represents a subgroup with distinct clinical presentations, prognosis, and management requirements. We investigated the survival, prognostic factors, and real-world treatment of NSCLC patients with EGFR mutation in clinical practice.
Methods
A retrospective review of all specimens sent for EGFR analysis from December 2009 to September 2015 was performed. Patient demographics, specimen type, EGFR mutation status/type, stage at diagnosis, treatment, response rate, and survival data were recorded.
Results
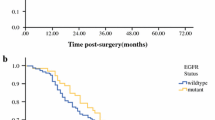
27/334 (8%) patient specimens sent for EGFR testing tested positive for a sensitising EGFR mutation. The median age was 65 years (40–85 years). Exon 19 deletion represented the most commonly detected alteration, accounting for 39% (n = 11). First-line treatment for those with Exon 18, 19, or 21 alterations (n = 24) was with an EGFR tyrosine kinase inhibitor (TKI) in 79% (n = 19). Objective response rate among these patients was 74% and median duration of response was 13 months (range 7–35 months).
Conclusion
The incidence of EGFR mutation in our cohort of NSCLC is 9% which is consistent with mutation incidence reported in other countries. The rate of EGFR mutation in our population is slightly below that reported internationally, but treatment outcomes are consistent with published data. Real-world patient data have important contributions to make with regard to quality measurement, incorporating patient experience into guidelines and identifying safety signals.
Similar content being viewed by others
References
National Cancer Registry (2015) Cancer in Ireland 1994–2013: annual report of the National Cancer Registry. National Cancer Registry, Cork
Reiss JW, Gandara D (2015) Lung cancer. In: Loprinzi CL (ed) ASCO-SEP medical oncology self-evaluation program. American Society of Clinical Oncology, Alexandria
Shim HS, da Lee H, Park EJ, Kim SH (2011) Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 135(10):1329–1334
Shikhrakab H, Elamin YY, O’Brien C, Gately K, Finn S, O’Byrne K, Osman N (2014) Epidermal growth factor receptor (EGFR) mutation testing, from bench to practice: a single institute experience. Ir Med J 107(7):201–204
Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O’Byrne K, Feng J, Lu S, Huang Y, Geater SL, Lee KY, Tsai CM, Gorbunova V, Hirsh V, Bennouna J, Orlov S, Mok T, Boyer M, Su WC, Lee KH, Kato T, Massey D, Shahidi M, Zazulina V, Sequist LV (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16(2):141–151
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was not funded.
This research has been carried out in compliance with ethical standards.
Conflict of interest
There are no conflicts of interest to declare. D. Kelly declares that she has no conflict of interest. L. Mc Sorley declares that she has no conflict of interest. E. O’Shea declares that he has no conflict of interest. E. Mc Carthy declares that she has no conflict of interest. S. Bowe declares that she has no conflict of interest. C. Brad y declares that she has no conflict of interest. J. Sui declares that she has no conflict of interest. M. A. Dawod declares that he has no conflict of interest. O. O’Brien declares that he has no conflict of interest. D. Graham declares that she has no conflict of interest. J. McCarthy declares that she has no conflict of interest. L. Burke declares that she has no conflict of interest. D. Power declares that he has no conflict of interest. S. O’Reilly declares that he has no conflict of interest. R. M. Bambury declares that he has no conflict of interest. D. O. Mahony declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
D. Kelly and L. Mc Sorley equally contributed to this work.
Rights and permissions
About this article
Cite this article
Kelly, D., Mc Sorley, L., O’Shea, E. et al. A regional analysis of epidermal growth factor receptor (EGFR) mutated lung cancer for HSE South. Ir J Med Sci 186, 855–857 (2017). https://doi.org/10.1007/s11845-017-1579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-017-1579-y