Abstract
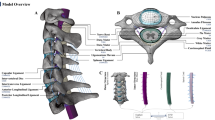
Recent studies have emphasized the importance of dynamic activity in the development of myelopathy. However, current knowledge of how degenerative factors affect the spinal cord during motion is still limited. This study aimed to investigate the effect of various types of preexisting herniated cervical disc and the ligamentum flavum ossification on the spinal cord during cervical flexion and extension. A detailed dynamic fluid-structure interaction finite element model of the cervical spine with the spinal cord was developed and validated. The changes of von Mises stress and maximum principal strain within the spinal cord in the period of normal, hyperflexion, and hyperextension were investigated, considering various types and grades of disc herniation and ossification of the ligamentum flavum. The flexion and extension of the cervical spine with spinal canal encroachment induced high stress and strain inside the spinal cord, and this effect was also amplified by increased canal encroachments and cervical hypermobility. The spinal cord might evade lateral encroachment, leading to a reduction in the maximum stress and principal strain within the spinal cord in local-type herniation. Although the impact was limited in the case of diffuse type, the maximum stress tended to appear in the white matter near the encroachment site while compression from both ventral and dorsal was essential to make maximum stress appear in the grey matter. The existence of canal encroachment can reduce the safe range for spinal cord activities, and hypermobility activities may induce spinal cord injury. Besides, the ligamentum flavum plays an important role in the development of central canal syndrome.
Significance. This model will enable researchers to have a better understanding of the influence of cervical degenerative diseases on the spinal cord during extension and flexion.
Graphical abstract








Similar content being viewed by others
References
Davies BM, Mowforth OD, Smith EK, Kotter MR (2018) Degenerative cervical myelopathy. BMJ 360:186. https://doi.org/10.1136/bmj.k186
Pettersson K, Kärrholm J, Toolanen G, Hildingsson C (1995) Decreased width of the spinal canal in patients with chronic symptoms after whiplash injury. Spine 20:1664–1667. https://doi.org/10.1097/00007632-199508000-00003
Amirouche F, Solitro GF, Siemionow K et al (2015) Role of posterior elements in the disc bulging of a degenerated cervical spine. Int J Spine Surg 9:13. https://doi.org/10.14444/2013
Badhiwala JH, Ahuja CS, Akbar MA et al (2020) Degenerative cervical myelopathy — update and future directions. Nat Rev Neurol 16:108–124. https://doi.org/10.1038/s41582-019-0303-0
Barclay L, Lentin P, Bourke-Taylor H, McDonald R (2019) The experiences of social and community participation of people with non-traumatic spinal cord injury. Aust Occup Ther J 66:61–67. https://doi.org/10.1111/1440-1630.12522
Sparrey CJ, Manley GT, Keaveny TM (2009) Effects of white, grey, and pia mater properties on tissue level stresses and strains in the compressed spinal cord. J Neurotrauma 26:585–595. https://doi.org/10.1089/neu.2008.0654
Wang J-J (2021) The biomechanical effect of preexisting different types of disc herniation in cervical hyperextension injury. J Orthop Surg Res 16:527. https://doi.org/10.1186/s13018-021-02677-y
Scifert J, Totoribe K, Goel V, Huntzinger J (2002) Spinal cord mechanics during flexion and extension of the cervical spine: a finite element study. Pain Physician 5:394–400
Khuyagbaatar B, Kim K, Man Park W, Hyuk Kim Y (2016) Biomechanical behaviors in three types of spinal cord injury mechanisms. J Biomech Eng 138:081003. https://doi.org/10.1115/1.4033794
Theodore N (2020) Degenerative cervical spondylosis. N Engl J Med 383:159–168. https://doi.org/10.1056/NEJMra2003558
Yamazaki S, Kokubun S, Ishii Y (1976) Tanaka Y (2003) Courses of cervical disc herniation causing myelopathy or radiculopathy: an analysis based on computed tomographic discograms. Spine Phila Pa 28:1171–1175. https://doi.org/10.1097/01.brs.0000067262.69584.0a
Kokubun S, Tanaka Y (1995) Types of cervical disc herniation and relation to myelopathy and radiculopathy. J Back Musculoskelet Rehabil 5:145–154. https://doi.org/10.3233/BMR-1995-5207
Matsumoto M, Chiba K, Ishikawa M et al (2001) Relationships between outcomes of conservative treatment and magnetic resonance imaging findings in patients with mild cervical myelopathy caused by soft disc herniations. Spine Phila Pa 1976 26:1592–1598. https://doi.org/10.1097/00007632-200107150-00021
Kang Y-M (2014) Herniated intervertebral disk induces hypertrophy and ossification of ligamentum flavum. J Spinal Disord Tech 27:382–389. https://doi.org/10.1097/bsd.0b013e3182a26532
Zhang B, Chen G, Chen X et al (2021) Cervical ossification of ligamentum flavum: elaborating an underappreciated but occasional contributor to myeloradiculopathy in aging population based on synthesis of individual participant data. Clin Interv Aging 16:897–908. https://doi.org/10.2147/CIA.S313357
Kuh SU (2006) Contributing factors affecting the prognosis surgical outcome for thoracic OLF. Eur Spine J 15:485–491. https://doi.org/10.1007/s00586-005-0903-9
Coughlin DJ, Rymarczuk GN, Dirks MS (2016) Noncalcified hypertrophic ligamentum flavum causing severe cervical stenosis and myelopathy: case report and review of the literature. World Neurosurg 95:618.e21–618.e26. https://doi.org/10.1016/j.wneu.2016.08.035
Kang Y, Lee JW, Koh YH et al (2011) New MRI grading system for the cervical canal stenosis. Am J Roentgenol 197:W134–W140. https://doi.org/10.2214/AJR.10.5560
Garay RS, Solitro GF, Lam KC et al (2022) Characterization of regional variation of bone mineral density in the geriatric human cervical spine by quantitative computed tomography. PLOS ONE 17:e0271187. https://doi.org/10.1371/journal.pone.0271187
Li Y, Chen Q, Shu X et al (2023) Biomechanical effect of osteoporosis on adjacent segments after anterior cervical corpectomy and fusion. World Neurosurg 171:e432–e439. https://doi.org/10.1016/j.wneu.2022.12.035
Sayit E (2013) Dynamic changes of the ligamentum flavum in the cervical spine assessed with kinetic magnetic resonance imaging. Glob Spine J 3:69–73. https://doi.org/10.1055/s-0033-1337121
Kwon S (2018) Analysis of dural sac thickness in the human cervical spine. Anat Sci Int 93:284–290. https://doi.org/10.1007/s12565-017-0412-z
Yan Y-B, Qi W, Wu Z-X et al (2012) Finite element study of the mechanical response in spinal cord during the thoracolumbar burst fracture. PLoS ONE 7:e41397. https://doi.org/10.1371/journal.pone.0041397
Cai X-Y, YuChi C-X, Du C-F, Mo Z-J (2020) The effect of follower load on the range of motion, facet joint force, and intradiscal pressure of the cervical spine: a finite element study. Med Biol Eng Comput 58:1695–1705. https://doi.org/10.1007/s11517-020-02189-7
Lee SCM, Lueck CJ (2014) Cerebrospinal fluid pressure in adults. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 34:278–283. https://doi.org/10.1097/WNO.0000000000000155
Persson C, Summers J, Hall RM (2011) The importance of fluid-structure interaction in spinal trauma models. J Neurotrauma 28:113–125. https://doi.org/10.1089/neu.2010.1332
Ichihara K, Taguchi T, Shimada Y et al (2001) Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J Neurotrauma 18:361–367. https://doi.org/10.1089/08977150151071053
Zhang QH, Teo EC, Ng HW (2005) Development and validation of a C0-C7 FE complex for biomechanical study. J Biomech Eng 127:729–735. https://doi.org/10.1115/1.1992527
Ruan JS, Khalil T, King AI (1991) Human head dynamic response to side impact by finite element modeling. J Biomech Eng 113:276–283. https://doi.org/10.1115/1.2894885
Ozawa H, Matsumoto T, Ohashi T et al (2004) Mechanical properties and function of the spinal pia mater. J Neurosurg Spine 1:122–127. https://doi.org/10.3171/spi.2004.1.1.0122
Czyz M, Scigala K, Jarmundowicz W, Beidziński R (2008) The biomechanical analysis of the traumatic cervical spinal cord injury using finite element approach. Acta Bioeng Biomech 10:43–54
Persson C, Evans S, Marsh R et al (2010) Poisson’s ratio and strain rate dependency of the constitutive behavior of spinal dura mater. Ann Biomed Eng 38:975–983. https://doi.org/10.1007/s10439-010-9924-6
Sugimoto T (2013) Ultrasonographic reference sizes of the median and ulnar nerves and the cervical nerve roots in healthy Japanese adults. Ultrasound Med Biol 39:1560–1570. https://doi.org/10.1016/j.ultrasmedbio.2013.03.031
Mihara A (2021) Tensile test of human lumbar ligamentum flavum: age-related changes of stiffness. Appl Sci 11:3337. https://doi.org/10.3390/app11083337
Brydon HL, Hayward R, Harkness W, Bayston R (1995) Physical properties of cerebrospinal fluid of relevance to shunt function. 1: The effect of protein upon CSF viscosity. Br J Neurosurg 9:639–644. https://doi.org/10.1080/02688699550040927
Richardson MG, Wissler RN (1996) Density of lumbar cerebrospinal fluid in pregnant and nonpregnant humans. Anesthesiology 85:326–330. https://doi.org/10.1097/00000542-199608000-00014
Bloomfield IG, Johnston IH, Bilston LE (1998) Effects of proteins, blood cells and glucose on the viscosity of cerebrospinal fluid. Pediatr Neurosurg 28:246–251. https://doi.org/10.1159/000028659
Lowrance EW, Latimer HB (1967) Weights and variability of components of the human vertebral column. Anat Rec 159:83–88. https://doi.org/10.1002/ar.1091590112
Singh A, Lu Y, Chen C, Cavanaugh JM (2006) Mechanical properties of spinal nerve roots subjected to tension at different strain rates. J Biomech 39:1669–1676. https://doi.org/10.1016/j.jbiomech.2005.04.023
Yoganandan N, Kumaresan S, Pintar FA (2000) Geometric and mechanical properties of human cervical spine ligaments. J Biomech Eng 122:623–629. https://doi.org/10.1115/1.1322034
Yoganandan N, Kumaresan S, Pintar FA (2001) Biomechanics of the cervical spine part 2. Cervical spine soft tissue responses and biomechanical modeling. Clin Biomech Bristol Avon 16:1–27. https://doi.org/10.1016/s0268-0033(00)00074-7
Hong-Wan N, Ee-Chon T, Qing-Hang Z (2004) Biomechanical effects of C2–C7 intersegmental stability due to laminectomy with unilateral and bilateral facetectomy. Spine 29:1737–1745. https://doi.org/10.1097/01.BRS.0000134574.36487.EB
Polak K, Czyż M, Ścigała K et al (2014) Biomechanical characteristics of the porcine denticulate ligament in different vertebral levels of the cervical spine—preliminary results of an experimental study. J Mech Behav Biomed Mater 34:165–170. https://doi.org/10.1016/j.jmbbm.2014.02.010
Shim VPW, Liu JF, Lee VS (2006) A technique for dynamic tensile testing of human cervical spine ligaments. Exp Mech 46:77–89. https://doi.org/10.1007/s11340-006-5865-2
Xu M, Zeng H, Zheng L et al (2022) Effect of degenerative factors on cervical spinal cord during flexion and extension: a dynamic finite element analysis. Biomech Model Mechanobiol 21:1743–1759. https://doi.org/10.1007/s10237-022-01617-x
Persson C, McLure SWD, Summers J, Hall RM (2009) The effect of bone fragment size and cerebrospinal fluid on spinal cord deformation during trauma: an ex vivo study: Laboratory investigation. J Neurosurg Spine 10:315–323. https://doi.org/10.3171/2009.1.SPINE08286
Lee SCM, Lueck CJ (2014) Cerebrospinal fluid pressure in adults. J Neuroophthalmol 34:278–283. https://doi.org/10.1097/wno.0000000000000155
Jones CF, Kroeker SG, Cripton PA, Hall RM (2008) The effect of cerebrospinal fluid on the biomechanics of spinal cord: an ex vivo bovine model using bovine and physical surrogate spinal cord. Spine 33:E580–E588. https://doi.org/10.1097/BRS.0b013e31817ecc57
Puglisi F, Ridi R, Cecchi F et al (2004) Segmental vertebral motion in the assessment of neck range of motion in whiplash patients. Int J Legal Med 118. https://doi.org/10.1007/s00414-004-0462-3
Mann E, Peterson CK, Hodler J (2011) Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine Phila Pa 1976 36:1081–1085. https://doi.org/10.1097/brs.0b013e3181ef6a1e
Song K-J, Choi B-W, Kim G-H, Kim J-R (2009) Clinical usefulness of CT-myelogram comparing with the MRI in degenerative cervical spinal disorders: is CTM still useful for primary diagnostic tool? J Spinal Disord Tech 22:353–357. https://doi.org/10.1097/BSD.0b013e31817df78e
Li Y, Fredrickson V, Resnick DK (2015) How should we grade lumbar disc herniation and nerve root compression? A systematic review. Clin Orthop 473:1896–1902. https://doi.org/10.1007/s11999-014-3674-y
Su Z-H, Liu J, Yang M-S et al (2022) Automatic grading of disc herniation, central canal stenosis and nerve roots compression in lumbar magnetic resonance image diagnosis. Front Endocrinol 13:890371. https://doi.org/10.3389/fendo.2022.890371
Maikos JT, Qian Z, Metaxas D, Shreiber DI (2008) Finite element analysis of spinal cord injury in the rat. J Neurotrauma 25:795–816. https://doi.org/10.1089/neu.2007.0423
Watabe N, Tominaga T, Shimizu H et al (1999) Quantitative analysis of cerebrospinal fluid flow in patients with cervical spondylosis using cine phase-contrast magnetic resonance imaging. Neurosurgery 44:779–784. https://doi.org/10.1097/00006123-199904000-00052
Martin BA, Kalata W, Loth F et al (2005) Syringomyelia hydrodynamics: an in vitro study based on in vivo measurements. J Biomech Eng 127:1110–1120. https://doi.org/10.1115/1.2073687
Pahlavian SH (2016) Accuracy of 4D flow measurement of cerebrospinal fluid dynamics in the cervical spine: an in vitro verification against numerical simulation. Ann Biomed Eng 44:3202–3214. https://doi.org/10.1007/s10439-016-1602-x
Heidari Pahlavian S, Bunck AC, Thyagaraj S et al (2016) Accuracy of 4D flow measurement of cerebrospinal fluid dynamics in the cervical spine: an in vitro verification against numerical simulation. Ann Biomed Eng 44:3202–3214. https://doi.org/10.1007/s10439-016-1602-x
Muhle C (1998) Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol 19:1763–1771
Bain AC, Meaney DF (2000) Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J Biomech Eng 122:615–622. https://doi.org/10.1115/1.1324667
Ouyang H, Galle B, Li J et al (2008) Biomechanics of spinal cord injury: a multimodal investigation using ex vivo guinea pig spinal cord white matter. J Neurotrauma 25:19–29. https://doi.org/10.1089/neu.2007.0340
Russell CM, Choo AM, Tetzlaff W et al (2012) Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J Neurotrauma 29:1574–1585. https://doi.org/10.1089/neu.2011.2225
Fiford RJ, Bilston LE (2005) The mechanical properties of rat spinal cord in vitro. J Biomech 38:1509–1515. https://doi.org/10.1016/j.jbiomech.2004.07.009
Chang G-L, Hung T-K, Feng WW (1988) An in-vivo measurement and analysis of viscoelastic properties of the spinal cord of cats. J Biomech Eng 110:115–122. https://doi.org/10.1115/1.3108415
Zheng L, Cao Y, Yang Y et al (2023) Effect of different types of ossification of the posterior longitudinal ligament on the dynamic biomechanical response of the spinal cord: a finite element analysis. J Biomech Eng 145:121002. https://doi.org/10.1115/1.4063194
Schmidt H, Shirazi-Adl A, Galbusera F, Wilke H-J (2010) Response analysis of the lumbar spine during regular daily activities—a finite element analysis. J Biomech 43:1849–1856. https://doi.org/10.1016/j.jbiomech.2010.03.035
Schmidt H, Galbusera F, Rohlmann A, Shirazi-Adl A (2013) What have we learned from finite element model studies of lumbar intervertebral discs in the past four decades? J Biomech 46:2342–2355. https://doi.org/10.1016/j.jbiomech.2013.07.014
Cappetti N, Naddeo A, Naddeo F, Solitro GF (2016) Finite elements/Taguchi method based procedure for the identification of the geometrical parameters significantly affecting the biomechanical behavior of a lumbar disc. Comput Methods Biomech Biomed Engin 19:1278–1285. https://doi.org/10.1080/10255842.2015.1128529
Stoner KE, Abode-Iyamah KO, Magnotta VA et al (2019) Measurement of in vivo spinal cord displacement and strain fields of healthy and myelopathic cervical spinal cord. J Neurosurg Spine 31:53–59. https://doi.org/10.3171/2018.12.SPINE18989
Rao SK, Wasyliw C, Nunez DB (2005) Spectrum of imaging findings in hyperextension injuries of the neck. RadioGraphics 25:1239–1254. https://doi.org/10.1148/rg.255045162
Schneider RC, Cherry G, Pantek H (1954) The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg 11:546–577. https://doi.org/10.3171/jns.1954.11.6.0546
Paholpak P, Nazareth A, Barkoh K et al (2017) Space available for cord, motion, and disc degeneration at the adjacent segments level of degenerative cervical spondylolisthesis using kinematic MRI. J Clin Neurosci 45:89–99. https://doi.org/10.1016/j.jocn.2017.07.029
Nouri A, Tetreault L, Singh A et al (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine 40:E675–E693. https://doi.org/10.1097/BRS.0000000000000913
Ames CP, Blondel B, Scheer JK et al (2013) Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine 38:S149–S160. https://doi.org/10.1097/BRS.0b013e3182a7f449
Pickett GE, Campos-Benitez M, Keller JL, Duggal N (2006) Epidemiology of traumatic spinal cord injury in Canada. Spine 31:799–805. https://doi.org/10.1097/01.brs.0000207258.80129.03
Chen X, Li L, Guo J et al (2016) Treadmill running exercise prevents senile osteoporosis and upregulates the Wnt signaling pathway in SAMP6 mice. Oncotarget 7:71072–71086. https://doi.org/10.18632/oncotarget.12125
Khan MH, Smith PN, Donaldson WF (2005) Acute quadriparesis caused by calcification of the entire cervical ligamentum flavum in a white female--report of an unusual case and a brief review of the literature: case report. Spine 30:E687–E691. https://doi.org/10.1097/01.brs.0000186472.88141.38
Singhal U, Jain M, Jaiswal AK, Behari S (2009) Unilateral ossified ligamentum flavum in the high cervical spine causing myelopathy. Indian J Orthop 43:305–308. https://doi.org/10.4103/0019-5413.49385
Jokich PM, Rubin JM, Dohrmann GJ (1984) Intraoperative ultrasonic evaluation of spinal cord motion. J Neurosurg 60:707–711. https://doi.org/10.3171/jns.1984.60.4.0707
Wolf K, Hupp M, Friedl S et al (2018) In cervical spondylotic myelopathy spinal cord motion is focally increased at the level of stenosis: a controlled cross-sectional study. Spinal Cord 56:769–776. https://doi.org/10.1038/s41393-018-0075-1
Daniels AH, McDonald CL, Basques BA, Kuris EO (2022) Ossified ligamentum flavum: epidemiology, treatment, and outcomes. J Am Acad Orthop Surg 30:e842–e851. https://doi.org/10.5435/JAAOS-D-21-01253
Ichihara K, Taguchi T, Sakuramoto I et al (2003) Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg Spine 99:278–285. https://doi.org/10.3171/spi.2003.99.3.0278
Cheng S, Clarke EC, Bilston LE (2008) Rheological properties of the tissues of the central nervous system: a review. Med Eng Phys 30:1318–1337. https://doi.org/10.1016/j.medengphy.2008.06.003
Mattucci S, Speidel J, Liu J et al (2019) Basic biomechanics of spinal cord injury - how injuries happen in people and how animal models have informed our understanding. Clin Biomech Bristol Avon 64:58–68. https://doi.org/10.1016/j.clinbiomech.2018.03.020
Zhu L, Wei X, Wang S (2016) Does cervical spine manipulation reduce pain in people with degenerative cervical radiculopathy? A systematic review of the evidence, and a meta-analysis. Clin Rehabil 30:145–155. https://doi.org/10.1177/0269215515570382
Shah S, Haughton V, Río AM (2011) CSF flow through the upper cervical spinal canal in Chiari I malformation. AJNR Am J Neuroradiol 32:1149–1153. https://doi.org/10.3174/ajnr.a2460
Funding
This work was supported by the National Key R&D Program of China (No. 2020YFC2008703 and No. 2022YFF1202600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Tongji Hospital Institutional Review Board (Ref. 2020-KYSB-096), and written informed consent was obtained from the participant.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Ml., Yang, Yt., Zeng, Hz. et al. Finite element modeling and analysis of effect of preexisting cervical degenerative disease on the spinal cord during flexion and extension. Med Biol Eng Comput 62, 1089–1104 (2024). https://doi.org/10.1007/s11517-023-02993-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02993-x