Abstract
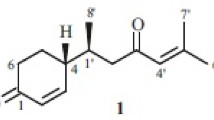
A novel C20 natural product, acacienone (1), was isolated from the leaves of Acacia mangium collected in Bangladesh. The structure of compound 1 was elucidated by spectral studies and X-ray crystallographic analysis. Acacienone (1) possesses a terpenoid-related tetracyclic framework containing 20 carbons with biogenetically unusual structural features: (i) vicinal C1-branches at the C-3 and C-4 positions in the A ring, and (ii) a cyclopentenone D ring in an androsterone-like assembly, lacking a methyl group at the C-13 position.







Similar content being viewed by others
References
Kusano R, Ogawa S, Matsuo Y, Tanaka T, Yazaki Y, Kouno I (2011) α-Amylase and lipase inhibitory activity and structural characterization of Acacia bark proanthocyanidins. J Nat Prod 74:119–128
Rifai Y, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M (2010) Terpenoids and a flavonoid glycoside from Acacia pennata leaves as Hedgehog/GLI-mediated transcriptional inhibitors. J Nat Prod 73:995–997
Mihara R, Barry KM, Mohammed CL, Mitsunaga T (2005) Comparison of antifungal and antioxidant activities of Acacia mangium and A. auriculiformis heartwood extracts. J Chem Ecol 31:789–804
Barry KM, Mihara R, Davies NW, Mitsunaga T, Mohammed CL (2005) Polyphenols in Acacia mangium and Acacia auriculiformis heartwood with reference to heart rot susceptibility. J Wood Sci 51:615–621
Zhang L, Chen J, Wang Y, Wu D, Xu M (2010) Phenolic extracts from Acacia mangium bark and their antioxidant activities. Molecules 15:3567–3577
Ishibashi M (2019) Screening for natural products that affect Wnt signaling activity. J Nat Med 73:697–705
Sato T, Arai MA, Yixizhuoma HY, Koyano T, Kowithayakorn T, Ishibashi M (2020) Cadinane sesquiterpenoids isolated from Santalum album using a screening program for Wnt signal inhibitory activity. J Nat Med 74:476–481
Karmakar UK, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M (2017) Boesenberols I-K, new isopimarane diterpenes from Boesenbergia pandurata with TRAIL-resistance overcoming activity. Tetrahedron Lett 58:3838–3841
Ahmed F, Ishibashi M (2016) Bio-active natural products with TRAIL-resistance overcoming activity. Chem Pharm Bull 64:119–127
Ohtani I, Kusumi T, Kashman Y, Kakisawa H (1991) High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J Am Chem Soc 113:4092–4096
Kubo I, Miura I, Pettei MJ, Lee Y, Pilkiewicz FG, Nakanishi K (1977) Muzigadial and warburganal, potent antifungal antiyeast, and African army worm antifeedant agents. Tetrahedron Lett 18:4553–4556
Corbett RE, Chee TL (1976) Extractives from Pseudowintera colorata. V. A new sesquiterpene lactone, colorata-4(13),8-dienolide. J Chem Soc Perkin Trans 1(8):850–857
Jansen BJM, Groot A (2004) Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat Prod Rep 21:449–477
Dewick PM (2001a) Medicinal natural products: a biosynthetic approach, 2nd edn. John Wiley & Sons Ltd., England, pp 234–235
Dewick PM (2001b) Medicinal natural products: a biosynthetic approach, 2nd edn. John Wiley & Sons Ltd., England, pp 277–278
Shimizu Y, Sato Y, Mitsuhashi H (1969) Isolation and characterization of fukujusonorone, an 18-norpregnane derivative from Adonis amurensis Regel et Radd. Experientia 25:1129–1130
Acknowledgements
This work was supported by KAKENHI Grant No. 20H03394 and 20K16024 from the Japan Society for the Promotion of Science. We would like to thank Dr. Tomoki Yoneda, Hokkaido University, for valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hara, Y., Totsugi, Y., Ichikawa, H. et al. Acacienone, a terpenoid-like natural product having an unprecedented C20 framework isolated from Acacia mangium leaves. J Nat Med 75, 99–104 (2021). https://doi.org/10.1007/s11418-020-01457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01457-y