Abstract
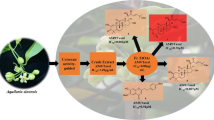
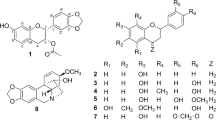
A methanol extract of the leaves of Cephalotaxus harringtonia var. nana and its ethyl acetate (EtOAc)-soluble fraction demonstrated strong antitumor activity against A549 and HT-29 cell lines. The EtOAc-soluble fraction was purified by column chromatography and high-performance liquid chromatography (HPLC) using a reverse-phase column to yield three novel acyl flavonoids and a biflavonoid, along with 15 other known compounds that included flavonoids, biflavonoids, and other phenolics. The structures of the new compounds were elucidated using spectral data from HR-MS and NMR, including two-dimensional NMR studies, as (2R,3R)-3-O-eicosanoyltaxifolin (1), (2R,3R)-3-O-docosanoyltaxifolin (2), (2R,3R)-3-O-tetracosanoyltaxifolin (3), and 6-methyl-4′,7,7″-tri-O-methylamentoflavone (4). The isolated compounds, including the known compounds, were tested for possible antitumor activity; some of the biflavones were found to be active. The potent antitumor activity of the extract was attributed to Cephalotaxus alkaloids, such as homoharringtonine (20).



Similar content being viewed by others
References
Kuroyanagi M, Shimomae M, Nagashima Y, Muto N, Okuda T, Kawahara N, Nakane T, Sano T (2005) New diarylheptanoids from Alnus japonica and their antioxidative activity. Chem Pharm Bull 53:1519–1523
Gao HY, Wu L, Kuroyanagi M, Harada K, Kawahara N, Nakane T, Umehara K, Hirasawa A, Nakamura Y (2003) Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells. Chem Pharm Bull 51:1318–1321
Kuroyanagi M, Ikeda R, Gao HY, Muto N, Otaki K, Sano T, Kawahara N, Nakane T (2008) Neurite outgrowth-promoting active constituents of the Japanese cypress (Chamaecyparis obtusa). Chem Pharm Bull 56:60–63
Takano I, Yasuda I, Nishijima M, Hitotsuyanagi Y, Takeya K, Itokawa H (1996) New oxygenated cephalotaxus alkaloids from Cephalotaxus harringtonia var. drupacea. J Nat Prod 59:1192–1195
Delfel NE, Rothfus JA (1977) Antitumor alkaloids in callus cultures of Cephalotaxus harringtonia. Phytochemistry 16:1595–1598
Bocar M, Jossang A, Bodo B (2003) New alkaloids from Cephalotaxus fortunei. J Nat Prod 66:152–154
Takano I, Yasuda I, Nishijima M, Hitotsuyanagi Y, Takeya K, Itokawa H (1996) Alkaloids from Cephalotaxus harringtonia. Phytochemistry 43:299–303
Kobayashi J, Yoshinaga M, Yoshida N, Shiro M, Morita H (2002) Cephalocyclidin A, a novel pentacyclic alkaloid from Cephalotaxus harringtonia var. nana. J Org Chem 67:2283–2286
Morita H, Yoshinaga M, Kobayashi J (2002) Cephalezomines G, H, J, K, L, and M, new alkaloids from Cephalotaxus harringtonia var. nana. Tetrahedron 58:5489–5495
Yoshinaga M, Morita H, Dota T, Kobayashi J (2004) Bis-cephalezomines A–E from Cephalotaxus harringtonia var. nana. Tetrahedron 60:7861–7868
Kuo YH, Lin CH, Hwang SY, Shen YC, Lee YL, Shyh-Yuan L (2000) A novel cytotoxic C-methylated biflavone from the stem of Cephalotaxus wilsoniana. Chem Pharm Bull 48:440–441
Miura H, Kawano N (1968) Sequoiaflavone in the leaves of Sequoia sempervirens and Cunninghamia lanceolata var. Konishii and its formation by partial demethylation. Yakugaku Zasshi 88:1489–1491
Markham KR, Sheppard C, Geiger H (1987) 13C-NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry 26:3335–3337
Li SH, Zhang HJ, Niu XM, Yao P, Sun HD, Fong HH (2003) Chemical constituents from Amentotaxus yunnanensis and Torreya yunnanensis. J Nat Prod 66:1002–1005
Ilyas N, Ilyas M, Rahman W, Okigawa M, Kawano N (1978) Biflavones from the leaves of Araucaria excelsa. Phytochemistry 17:987–990
Wu T, Abdulla R, Yang Y, Aisa A (2008) Flavonoids from Gossypium hirsutum flowers. Chem Nat Compd 44:370–371
Fang JM, Lee CK, Cheng YS (1992) Lignans from leaves of Juniperus chinensis. Phytochemistry 31:3659–3661
Powell RG, Weisleder D, Smith CR Jr, Rohwedder WK (1970) Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett 11:815–818
Gaffield W (1970) Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: determination of aglycone chirality in flavanone glycosides. Tetrahedron 26:4093–4108
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Komoto, N., Nakane, T., Matsumoto, S. et al. Acyl flavonoids, biflavones, and flavonoids from Cephalotaxus harringtonia var. nana . J Nat Med 69, 479–486 (2015). https://doi.org/10.1007/s11418-015-0912-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-015-0912-x