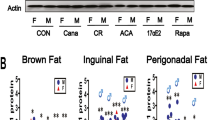
Abstract
Using mouse models and high-throughput proteomics, we conducted an in-depth analysis of the proteome changes induced in response to seven interventions known to increase mouse lifespan. This included two genetic mutations, a growth hormone receptor knockout (GHRKO mice) and a mutation in the Pit-1 locus (Snell dwarf mice), four drug treatments (rapamycin, acarbose, canagliflozin, and 17α-estradiol), and caloric restriction. Each of the interventions studied induced variable changes in the concentrations of proteins across liver, kidney, and gastrocnemius muscle tissue samples, with the strongest responses in the liver and limited concordance in protein responses across tissues. To the extent that these interventions promote longevity through common biological mechanisms, we anticipated that proteins associated with longevity could be identified by characterizing shared responses across all or multiple interventions. Many of the proteome alterations induced by each intervention were distinct, potentially implicating a variety of biological pathways as being related to lifespan extension. While we found no protein that was affected similarly by every intervention, we identified a set of proteins that responded to multiple interventions. These proteins were functionally diverse but tended to be involved in peroxisomal oxidation and metabolism of fatty acids. These results provide candidate proteins and biological mechanisms related to enhancing longevity that can inform research on therapeutic approaches to promote healthy aging.





Similar content being viewed by others
Data availability
All sample analysis run order files, mass spectrometry dia-PASEF raw folders corresponding to mouse tissue experiments (.d), FASTA database used to generate the assay libraries (.fasta), mouse hybrid spectral assay library (.txt) and its DIALib-QC report (.tsv), and three unnormalized peptide quantitation data files (.csv) for each tissue have been deposited with the ProteomeXchange Consortium via the PRIDE partner repository [50, 51] with the data set identifier PXD040497 (http://www.ebi.ac.uk/pride). Code describing our statistical data analysis that can be used to reproduce our main results can be found at https://github.com/longevity-consortium/M005MouseLongevityPaper2023.
References
Bartke A, Wright JC, Mattison JA, et al. Extending the lifespan of long-lived mice. Nature. 2001;414:412. https://doi.org/10.1038/35106646
Drake JC, Bruns DR, Peelor FF, et al. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14:474–82. https://doi.org/10.1111/acel.12329
Gesing A, Masternak MM, Lewinski A, et al. Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene–disrupted mice. J Gerontol: Series A. 2013;68:639–51. https://doi.org/10.1093/gerona/gls231
Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. https://doi.org/10.1038/nature08221
Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–82. https://doi.org/10.1111/acel.12170
Harrison DE, Strong R, Alavez S, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019;18:e12898. https://doi.org/10.1111/acel.12898
Miller RA, Harrison DE, Allison DB, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5 https://doi.org/10.1172/jci.insight.140019
Tyshkovskiy A, Bozaykut P, Borodinova AA, et al. Identification and application of gene expression signatures associated with lifespan extension. Cell Metab. 2019;30:573–593.e8. https://doi.org/10.1016/j.cmet.2019.06.018
Li X, Shi X, McPherson M, et al. Cap-independent translation of GPLD1 enhances markers of brain health in long-lived mutant and drug-treated mice. Aging Cell. 2022;21:e13685. https://doi.org/10.1111/acel.13685
Shen Z, Hinson A, Miller RA, Garcia GG. Cap-independent translation: a shared mechanism for lifespan extension by rapamycin, acarbose, and 17α-estradiol. Aging Cell. 2021;20:e13345. https://doi.org/10.1111/acel.13345
Ozkurede U, Kala R, Johnson C, et al. Cap-independent mRNA translation is upregulated in long-lived endocrine mutant mice. J Mol Endocrinol. 2019;63:123–38. https://doi.org/10.1530/JME-19-0021
Wink L, Miller RA, Garcia GG. Rapamycin, Acarbose and 17α-estradiol share common mechanisms regulating the MAPK pathways involved in intracellular signaling and inflammation. Immun Ageing. 2022;19:8. https://doi.org/10.1186/s12979-022-00264-1
Li X, Frazier JA, Spahiu E, et al. Muscle-dependent regulation of adipose tissue function in long-lived growth hormone-mutant mice. Aging. 2020;12:8766–89. https://doi.org/10.18632/aging.103380.
Li X, McPherson M, Hager M, et al. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. Geroscience. 2023; https://doi.org/10.1007/s11357-023-00770-0
Bonkowski MS, Rocha JS, Masternak MM, et al. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci. 2006;103:7901–5. https://doi.org/10.1073/pnas.0600161103
Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci. 2001;98:6736–41. https://doi.org/10.1073/pnas.111158898
Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2010;65:1275–84. https://doi.org/10.1093/gerona/glq155
Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–77. https://doi.org/10.1111/acel.12194
Dominick G, Berryman DE, List EO, et al. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology. 2015;156:565–75. https://doi.org/10.1210/en.2014-1690
Macchiarini F, Miller RA, Strong R, et al. Chapter 10 - NIA interventions testing program: a collaborative approach for investigating interventions to promote healthy aging. In: Musi N, Hornsby PJ, editors. Handbook of the Biology of Aging (Ninth Edition). Academic Press; 2021. p. 219–35.
Auer H, Mobley J, Ayers L, et al. The effects of frozen tissue storage conditions on the integrity of RNA and protein. Biotech Histochem. 2014;89:518–28. https://doi.org/10.3109/10520295.2014.904927
LaBaer J. Improving international research with clinical specimens: 5 achievable objectives. J Proteome Res. 2012;11:5592–601. https://doi.org/10.1021/pr300796m
Escher C, Reiter L, MacLean B, et al. Using iRT, a normalized retention time for more targeted measurement of peptides. Proteomics. 2012;12:1111–21. https://doi.org/10.1002/pmic.201100463
Bruderer R, Bernhardt OM, Gandhi T, Reiter L. High-precision iRT prediction in the targeted analysis of data-independent acquisition and its impact on identification and quantitation. Proteomics. 2016;16:2246–56. https://doi.org/10.1002/pmic.201500488
Krasny L, Bland P, Burns J, et al. A mouse SWATH-mass spectrometry reference spectral library enables deconvolution of species-specific proteomic alterations in human tumour xenografts. Dis Model Mech. 2020;13:dmm044586. https://doi.org/10.1242/dmm.044586
Midha MK, Campbell DS, Kapil C, et al. DIALib-QC an assessment tool for spectral libraries in data-independent acquisition proteomics. Nat Commun. 2020;11:5251. https://doi.org/10.1038/s41467-020-18901-y
Bland JM, Altman DG. Statistics notes: calculating correlation coefficients with repeated observations: Part 1—correlation within subjects. BMJ. 1995;310:446. https://doi.org/10.1136/bmj.310.6977.446
Bakdash JZ, Marusich LR (2022) rmcorr: repeated measures correlation
Lazar C, Gatto L, Ferro M, et al. Accounting for the multiple natures of missing values in label-free quantitative proteomics data sets to compare imputation strategies. J Proteome Res. 2016;15:1116–25. https://doi.org/10.1021/acs.jproteome.5b00981
McGurk KA, Dagliati A, Chiasserini D, et al. The use of missing values in proteomic data-independent acquisition mass spectrometry to enable disease activity discrimination. Bioinformatics. 2020;36:2217–23. https://doi.org/10.1093/bioinformatics/btz898
Cragg JG. Some statistical models for limited dependent variables with application to the demand for durable goods. Econometrica. 1971;39:829–44. https://doi.org/10.2307/1909582
StataCorp (2021) Stata statistical software
Sellke T, Bayarri MJ, Berger JO. Calibration of ρ values for testing precise null hypotheses. Am Stat. 2001;55:62–71. https://doi.org/10.1198/000313001300339950
Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–45. https://doi.org/10.1101/gr.6202607
Carlson, M (2019) org.Mm.eg.db: Genome wide annotation for Mouse
Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. https://doi.org/10.1089/omi.2011.0118
Voichita C, Donato M, Draghici S. Incorporating gene significance in the impact analysis of signaling pathways. In: 2012 11th International Conference on Machine Learning and Applications; 2012. p. 126–31.
Voichita C, Ansari S, Draghici S (2022) ROntoTools: R onto-tools suite.
Kanehisa M, Furumichi M, Sato Y, et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–92. https://doi.org/10.1093/nar/gkac963
Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–95. https://doi.org/10.1152/ajpendo.00110.2012
Li X, Bartke A, Berryman DE, et al. Direct and indirect effects of growth hormone receptor ablation on liver expression of xenobiotic metabolizing genes. Am J Physiol Endocrinol Metab. 2013;305:E942–50. https://doi.org/10.1152/ajpendo.00304.2013
Herrera JJ, Louzon S, Pifer K, et al. Acarbose has sex-dependent and -independent effects on age-related physical function, cardiac health, and lipid biology. JCI Insight. 5:e137474. https://doi.org/10.1172/jci.insight.137474
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. https://doi.org/10.1038/ncb2329
Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16:652–60. https://doi.org/10.1111/acel.12590
Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–112. https://doi.org/10.1016/j.cmet.2016.05.027
Takemon Y, Chick JM, Gerdes Gyuricza I, et al. Proteomic and transcriptomic profiling reveal different aspects of aging in the kidney. Elife. 2021;10:e62585. https://doi.org/10.7554/eLife.62585
Gerdes Gyuricza I, Chick JM, Keele GR, et al. Genome-wide transcript and protein analysis highlights the role of protein homeostasis in the aging mouse heart. Genome Res. 2022;32:838–52. https://doi.org/10.1101/gr.275672.121
Orwoll ES, Wiedrick J, Nielson CM, et al. Proteomic assessment of serum biomarkers of longevity in older men. Aging Cell. 2020;19:e13253. https://doi.org/10.1111/acel.13253
Sebastiani P, Federico A, Morris M, et al. Protein signatures of centenarians and their offspring suggest centenarians age slower than other humans. Aging Cell. 2021;20:e13290. https://doi.org/10.1111/acel.13290
Vizcaíno JA, Côté R, Reisinger F, et al. A guide to the proteomics identifications database proteomics data repository. Proteomics. 2009;9:4276–83. https://doi.org/10.1002/pmic.200900402
Perez-Riverol Y, Bai J, Bandla C, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–52. https://doi.org/10.1093/nar/gkab1038
Acknowledgements
We thank Kelly Crebs for excellent technical assistance.
Funding
This work was funded in part by the National Institutes of Health grants, from the National Institute of General Medical Sciences R01 GM087221, the Office of the Director S10OD026936, the National Institute on Aging U19AG023122, and the National Science Foundation award 1920268.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

ESM 1
Supplemental Figure 1: (A) Venn diagram of the number of proteins detected in each tissue. (B) Dendrogram of similarity in protein abundances across all tissues. (PNG 108 kb)

ESM 2
Supplemental Figure 2: (A-C) Scatterplots comparing mean protein abundances between liver and kidney (A), liver and muscle (B), and kidney and muscle (C) samples across mice of both sexes. (D-F) Scatterplot comparing mean protein abundances between female and male samples in liver (D), kidney (E), and muscle (F) tissues. (PNG 342 kb)

ESM 3
Supplemental Figure 3: Variation in protein abundances explained by mouse background, sex, and longevity intervention for each quantified protein in liver (A), kidney (B), and muscle (C) tissues. Results from fitting a random effects model with intervention nested within background for each protein individually. (D) The abundances by intervention and sex of proteins for which the percent variation explained by intervention was greater than 60% in liver samples. (PNG 746 kb)

ESM 4
Supplemental Figure 4: The log2 fold-change and log10 Bayes factor for proteins within each treatment compared to controls for male (A) and female (B) kidney samples. Dashed vertical lines correspond to a log2 fold-change of +/- 0.5 while the dashed vertical line corresponds to a Bayes factor of 10. (PNG 569 kb)

ESM 5
Supplemental Figure 5: The log2 fold-change and log10 Bayes factor for proteins within each treatment compared to controls for male (A) and female (B) muscle samples. Dashed vertical lines correspond to a log2 fold-change of +/- 0.5 while the dashed vertical line corresponds to a Bayes factor of 10. (PNG 464 kb)

ESM 6
Supplemental Figure 6: Fold-changes in response to each longevity intervention in females (x-axis) compared to males (y-axis) in liver (A), kidney (B), and muscle (C) tissues. Dashed lines indicate log2 fold-changes of +/- 0.5 in males (horizontal) and females (vertical), while the diagonal dotted line marks a 1:1 relationship. Points are colored blue if the protein exhibited an absolute log2 fold-change greater than 0.5 with a Bayes factor greater than 10 in both sexes, red if in males only, yellow if in females only, and grey if in neither. (PNG 1040 kb)

ESM 7
Supplemental Figure 7: (A) Changes in protein abundances across multiple longevity treatments in kidney. Proteins shown are limited to those exhibiting an absolute log2 fold-change ≥ 0.5 and a Bayes factor ≥ 10 in response to at least three interventions of either sex. The color of the points indicates the associated Bayes factor and the size is proportional to the magnitude of the fold-change with closed points indicating a positive change and open points indicating a negative change. (B) GO-Terms that are enriched (with an associated adjusted p-value ≤ 0.01) among proteins with shared responses across longevity promoting interventions in kidney. (PNG 1022 kb)

ESM 8
Supplemental Figure 8: Changes in protein abundances across multiple longevity treatments in muscle. Proteins shown are limited to those exhibiting an absolute log2 fold-change greater than ≥ 0.5 and a Bayes factor ≥ 10 in response to at least three interventions of either sex. The color of the points indicates the associated Bayes factor and the size is correlated with the magnitude of the fold-change with closed points indicating a positive change and open points indicating a negative change. No GO-Terms where are enriched with an associated adjusted p-value ≤ 0.01 among proteins with shared responses across longevity promoting interventions in muscle. (PNG 218 kb)

ESM 9
Supplemental Figure 9: Comparison of protein responses to longevity interventions in mouse livers to protein differences between long-lived humans and not in the MrOS study for proteins detected in both datasets for liver, kidney, and muscle tissues. The color of the points indicates the associated Bayes factor and the size is proportional to the magnitude of the fold-change with closed points indicating a positive change and open points indicating a negative change. (PNG 1008 kb)

ESM 10
Supplemental Figure 10: Protein responses to longevity-promoting intervention-by-sex groups in mouse liver, kidney, and muscle for proteins also differentially abundant in centenarians in NECS. Proteins shown are limited to those that exhibit both differential abundance in centenarian humans compared to controls with an adjusted p-value less than 0.001 in NECS and exhibited an absolute log2 fold-change ≥ 0.5 and a Bayes factor ≥ 10 in response to at least one interventions of either sex in mice. The color of the points indicates the associated Bayes factor and the size is proportional to the magnitude of the fold-change with closed points indicating a positive change and open points indicating a negative change. (PNG 1382 kb)
ESM 11
(CSV 4602 kb)
ESM 12
(CSV 5249 kb)
ESM 13
(CSV 2807 kb)
ESM 14
(CSV 119 kb)
ESM 15
(CSV 87 kb)
ESM 16
(CSV 36 kb)
ESM 17
(CSV 6 kb)
ESM 18
(PDF 644 kb)
About this article
Cite this article
Burns, A.R., Wiedrick, J., Feryn, A. et al. Proteomic changes induced by longevity-promoting interventions in mice. GeroScience 46, 1543–1560 (2024). https://doi.org/10.1007/s11357-023-00917-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00917-z