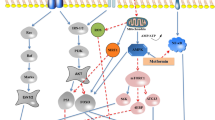
Abstract
Average and maximal lifespan can be increased in mice, in one or both sexes, by four drugs: rapamycin, acarbose, 17a-estradiol, and canagliflozin. We show here that these four drugs, as well as a calorie-restricted diet, can induce a common set of changes in fat, macrophages, plasma, muscle, and brain when evaluated in young adults at 12 months of age. These shared traits include an increase in uncoupling protein UCP1 in brown fat and in subcutaneous and intra-abdominal white fat, a decline in proinflammatory M1 macrophages and corresponding increase in anti-inflammatory M2 macrophages, an increase in muscle fibronectin type III domain containing 5 (FNDC5) and its cleavage product irisin, and higher levels of doublecortin (DCX) and brain-derived neurotrophic factor (BDNF) in brain. Each of these proteins is thought to play a role in one or more age-related diseases, including metabolic, inflammatory, and neurodegenerative diseases. We have previously shown that the same suite of changes is seen in each of four varieties of slow-aging single-gene mutant mice. We propose that these changes may be a part of a shared common pathway that is seen in slow-aging mice whether the delayed aging is due to a mutation, a low-calorie diet, or a drug.






Similar content being viewed by others
Data availability
All raw images, densitometric data, and statistical calculations are available from the authors (XL, RAM) on request.
References
Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Sci. 2010;328(5976):321–6.
Ravussin E, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–104.
Mattison JA, et al. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063.
Miller RA, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21).
Harrison DE, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–82.
Strong R, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84.
Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–5.
Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201.
Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77.
Ohgaki R, et al. Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2. J Pharmacol Exp Ther. 2016;358(1):94–102.
Nomura S, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53(17):6355–60.
Kapahi P, Kaeberlein M, Hansen M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res Rev. 2017;39:3–14.
Flanagan EW, et al. Calorie Restriction and aging in humans. Annu Rev Nutr. 2020;40:105–33.
Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–45.
Lashinger LM, et al. Rapamycin partially mimics the anticancer effects of calorie restriction in a murine model of pancreatic cancer. Cancer Prev Res. 2011;4(7):1041–51.
Hussein O, et al. Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett. 2012;314(2):176–84.
Zhao L, et al. Low-dose oral sirolimus reduces atherogenesis, vascular inflammation and modulates plaque composition in mice lacking the LDL receptor. Br J Pharmacol. 2009;156(5):774–85.
Jahrling JB, et al. mTOR drives cerebral blood flow and memory deficits in LDLR(-/-) mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab. 2018;38(1):58–74.
Tramutola A, et al. Intranasal rapamycin ameliorates Alzheimer-like cognitive decline in a mouse model of Down syndrome. Transl Neurodegener. 2018;7:28.
Madar Z, Hazan A. Effect of miglitol and acarbose on starch digestion, daily plasma glucose profiles and cataract formation. J Basic Clin Physiol Pharmacol. 1993;4(1–2):69–81.
Madar Z, Hazan A, Pollack A. Beneficial effects of acarbose on daily plasma glucose profile and cataract development in sand rats. Eye (Lond). 1994;8(Pt 3):353–6.
Wolf T, et al. The MalR type regulator AcrC is a transcriptional repressor of acarbose biosynthetic genes in Actinoplanes sp. SE50/110. BMC Genomics. 2017;18(1):562.
Shen Z, et al. Cap-independent translation: a shared mechanism for lifespan extension by rapamycin, acarbose, and 17α-estradiol. Aging Cell. 2021;20(5):e13345.
Wink L, Miller RA, Garcia GG. Rapamycin, Acarbose and 17α-estradiol share common mechanisms regulating the MAPK pathways involved in intracellular signaling and inflammation. Immun Ageing. 2022;19(1):8.
Graja A, Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontol. 2015;61(3):211–7.
Rogers NH, et al. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11(6):1074–83.
Ricquier D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: a historical perspective. Front Endocrinol. 2011;2:85.
Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature. 1997;387(6628):94–7.
Aquila H, Link TA, Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. Embo J. 1985;4(9):2369–76.
Heaton GM, et al. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur J Biochem. 1978;82(2):515–21.
Vitali A, et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619–29.
Petrovic N, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–64.
Ishibashi J, Seale P. Medicine. Beige can be slimming Science. 2010;328(5982):1113–4.
Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359.
Bordicchia M, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–36.
Cederberg A, et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106(5):563–73.
Seale P, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105.
Boström P, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8.
Li X, et al. Muscle-dependent regulation of adipose tissue function in long-lived growth hormone-mutant mice. Aging. 2020;12(10):8766–89.
Li X, et al. Transient early life growth hormone exposure permanently alters brain, muscle, liver, macrophage, and adipocyte status in long-lived Ames dwarf mice. Faseb j. 2022;36(7):e22394.
Li X, et al. Recapitulation of anti-aging phenotypes by global, but not by muscle-specific, deletion of PAPP-A in mice. Geroscience. 2023;45(2):931–48.
Kraakman MJ, et al. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol. 2014;5:470.
Patsouris D, et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS ONE. 2014;9(10):e110653.
Fuentes L, Roszer T, Ricote M. Inflammatory mediators and insulin resistance in obesity: role of nuclear receptor signaling in macrophages. Mediators Inflamm. 2010;2010:219583.
Costantini A, et al. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging. 2018;10(6):1268–80.
Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32(1):18–26.
Mills CD, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73.
Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14(4):341–6.
Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunol. 2018;155(4):407–17.
Thomas D, Apovian C. Macrophage functions in lean and obese adipose tissue. Metab. 2017;72:120–43.
Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224(2):154–67.
Teufel A, et al. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297(1–2):79–83.
Huh JY, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metab. 2012;61(12):1725–38.
Mazur-Bialy AI, Pochec E, Zarawski M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017;18(4):701.
Xiong XQ, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metab. 2018;83:31–41.
Matsuo Y, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6(1):62–72.
Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736.
MacPherson REK. Filling the void: a role for exercise-induced BDNF and brain amyloid precursor protein processing. Am J Physiol Regul Integr Comp Physiol. 2017;313(5):R585-r593.
Bednarski E, et al. Lysosomal dysfunction reduces brain-derived neurotrophic factor expression. Exp Neurol. 1998;150(1):128–35.
Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328(3):261–4.
Pignataro P, et al. FNDC5/irisin system in neuroinflammation and neurodegenerative diseases: update and novel perspective. Int J Mol Sci. 2021;22(4):1605.
Wrann CD, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–59.
Gleeson JG, et al. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–71.
Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–56.
Couillard-Despres S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14.
Balthazart, J. and G.F. Ball, Doublecortin is a highly valuable endogenous marker of adult neurogenesis in canaries. Commentary on Vellema M et al. (2014): evaluating the predictive value of doublecortin as a marker for adult neurogenesis in canaries (Serinus canaria) . J Comparative Neurol 522:1299–1315. Brain Behav Evol, 2014. 84(1): 1–4.
Li X, et al. Cap-independent translation of GPLD1 enhances markers of brain health in long-lived mutant and drug-treated mice. Aging Cell. 2022;21(9):e13685
Horowitz AM, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369(6500):167–73.
Wrann CD. FNDC5/irisin—their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015;1(1):55–61.
Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167(1):10–4.
Cousin B, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–42.
Stout MB, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging. 2014;6(7):575–86.
Darcy J, et al. Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinol. 2016;157(12):4744–53.
Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med. 2021;99(1):1–20.
Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15(9):507–24.
van der Heijden RA, et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging. 2015;7(4):256–68.
Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84.
Sadagurski M, Cady G, Miller RA. Anti-aging drugs reduce hypothalamic inflammation in a sex-specific manner. Aging Cell. 2017;16(4):652–60.
Jayarathne HSM, et al. Neuroprotective effects of Canagliflozin: lessons from aged genetically diverse UM-HET3 mice. Aging Cell. 2022;21(7):e13653.
Zhang Y, et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311(2):E530–41.
Liu P, et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor Neurol Neurosci. 2015;33(2):143–57.
Ozkurede U, et al. Cap-independent mRNA translation is upregulated in long-lived endocrine mutant mice. J Mol Endocrinol. 2019;63(2):123–38.
Dominick G, et al. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinol. 2015;156(2):565–75.
Acknowledgements
We thank Lori Roberts, Natalie Perry, Roxann Alonzo, Jacob Sheets, and Ilkim Erturk for expert assistance in animal care. The work was supported by a grant from the Glenn Foundation for Medical Research, and by NIH grants AG023122 and AG024824.
Author information
Authors and Affiliations
Contributions
X.L. designed and did the experiments and wrote the manuscript. M.M., M.L., M.H., and P.C. helped with experiments. P.C. did western blotting experiments. R.A.M. helped design the experiments and to edit the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Li, X., McPherson, M., Hager, M. et al. Four anti-aging drugs and calorie-restricted diet produce parallel effects in fat, brain, muscle, macrophages, and plasma of young mice. GeroScience 45, 2495–2510 (2023). https://doi.org/10.1007/s11357-023-00770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00770-0