Abstract
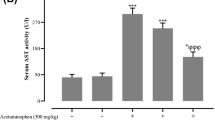
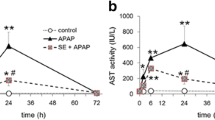
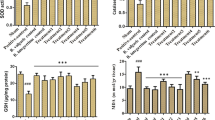
In the current report, we examined the potential beneficial role of soursop fruit extract (SSFE) on liver injury induced by a single paracetamol (APAP) overdose (2000 mg/kg). Thirty-five Wistar albino rats were randomly divided into five groups as follows: control, SSFE, APAP, SSFE+APAP, and silymarin (SIL)+APAP. APAP intoxication was found to elevate alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin levels. Moreover, it increased the levels of malondialdehyde, nitrites, and nitrates and depleted glutathione, superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase. APAP intoxication inactivated the nuclear factor erythroid 2-related factor 2 (Nrf2) defense pathway and upregulated the expression of heme oxygenase-1 (HO-1). APAP administration enhanced the activation of nuclear factor-kappa B (NF-κB), the elevation of tumor necrosis factor-alpha and interleukin 1-beta levels, and the upregulation of inducible nitric oxide synthase mRNA expression. In addition, APAP activated the overexpression of Bax protein, increased release of cytochrome c, and the downregulation of Bcl-2 protein. Finally, APAP-induced overexpression of transforming growth factor-beta (TGF-β) further suggested enhanced liver damage. On the other hand, SSFE pretreatment attenuated these biochemical, molecular, and histopathological alterations in the liver, which might be partially due to the regulation of hepatic Nrf2/HO-1 and downregulation of NF-κB and TGF-β.










Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- APAP:
-
Paracetamol
- AST:
-
Aspartate aminotransferase
- CAT:
-
Catalase
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- GSH-R:
-
Glutathione reductase
- HO-1:
-
Heme oxygenase-1
- IL-1β:
-
Interleukin-1 beta
- iNOS:
-
Inducible nitric oxide synthase
- MDA:
-
Malondialdehyde
- NAPQI:
-
N-acetyl-P-benzoquinone imine
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor erythroid 2–related factor 2
- SIL:
-
Silymarin
- SOD:
-
Superoxide dismutase
- SSFE:
-
Soursop fruit extract
- TGF-β:
-
tumor growth factor-beta
- TNF-α:
-
tumor necrosis factor-alpha
References
Adewole SO, Ojewole JA (2008) Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 6:30–41
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Almeer RS, Abdel Moneim AE (2018) Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxidative Med Cell Longev 2018:11
Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB, Abdel Moneim AE (2014) The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxidative Med Cell Longev 2014:381413
Araujo JA, Zhang M, Yin F (2012) Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3:119
Arthur F, Woode E, Larbie C (2012) Bilirubin lowering potential of Annona muricata (Linn.) in temporary jaundiced rats. Am J Pharmacol Toxicol 7(2):33–40
Bian X, Wang S, Liu J, Zhao Y, Li H, Zhang L, Li P (2018) Hepatoprotective effect of chiisanoside against acetaminophen-induced acute liver injury in mice. Nat Prod Res:1–4. https://doi.org/10.1080/14786419.2018.1460841
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI (2015) Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol 63:503–514
Chiu H, Brittingham JA, Laskin DL (2002) Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol 181:106–115
Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM (2012) Pathogenesis of liver injury in acute liver failure. Gastroenterology 143:e1–e7
Das J, Ghosh J, Manna P, Sil PC (2010) Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res 44:340–355
De Vega L, Fernandez RP, Mateo MC, Bustamante JB, Herrero AM, Munguira EB (2002) Glutathione determination and a study of the activity of glutathione-peroxidase, glutathione-transferase, and glutathione-reductase in renal transplants. Ren Fail 24:421–432
Dkhil M, Abdel Moneim A, Hafez T, Mubaraki M, Mohamed W, Thagfan F, Al-Quraishy S (2019) Myristica fragrans kernels prevent paracetamol-induced hepatotoxicity by inducing anti-apoptotic genes and Nrf2/HO-1 pathway. Int J Mol Sci 20(4):993
Dokumacioglu E, Iskender H, Aktas MS, Hanedan B, Dokumacioglu A, Sen TM, Musmul A (2017) The effect of sulforaphane on oxidative stress and inflammation in rats with toxic hepatitis induced by acetaminophene. Bratisl Lek Listy 118:453–459
Du K, Williams CD, McGill MR, Xie Y, Farhood A, Vinken M, Jaeschke H (2013) The gap junction inhibitor 2-aminoethoxy-diphenyl-borate protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes and c-jun N-terminal kinase activation. Toxicol Appl Pharmacol 273:484–491
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P (2016) TGF-beta signalling and liver disease. FEBS J 283:2219–2232
Fisher AEO, Maxwell SC, Naughton DP (2003) Catalase and superoxide dismutase mimics for the treatment of inflammatory diseases. Inorg Chem Commun 6:1205–1208
Florence NT, Benoit MZ, Jonas K, Alexandra T, Desire DD, Pierre K, Theophile D (2014) Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol 151:784–790
Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: a guide for clinicians. CMAJ 172:367–379
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Guan YS, He Q (2015) Plants consumption and liver health. Evid Based Complement Alternat Med 2015:824185
Guo LM, Liu JY, Xu DZ, Li BS, Han H, Wang LH, Zhang WY, Lu LH, Guo X, Sun FX, Zhang HY, Liu XD, Zhang JP, Yao Y, He ZP, Wang MM (2003) Application of molecular adsorbents recirculating system to remove NO and cytokines in severe liver failure patients with multiple organ dysfunction syndrome. Liver Int 23(Suppl 3):16–20
Hamid RA, Foong CP, Ahmad Z, Hussain MK (2012) Antinociceptive and anti-ulcerogenic activities of the ethanolic extract of Annona muricata leaf. Rev Bras 22:630–641
Hinson JA, Roberts DW, James LP (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405
Horng CT, Liu ZH, Huang YT, Lee HJ, Wang CJ (2017) Extract from Mulberry (Morus australis) leaf decelerate acetaminophen induced hepatic inflammation involving downregulation of myeloid differentiation factor 88 (MyD88) signals. J Food Drug Anal 25:862–871
Hu B, Colletti LM (2010) CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology 52:691–702
Jaeschke H, Knight TR, Bajt ML (2003) The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett 144:279–288
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Kaya H, Polat B, Albayrak A, Mercantepe T, Buyuk B (2018) Protective effect of an L-type calcium channel blocker, amlodipine, on paracetamol-induced hepatotoxicity in rats. Hum Exp Toxicol 37(11):1169–1179
Kim GT, Tran NK, Choi EH, Song YJ, Song JH, Shim SM, Park TS (2016) Immunomodulatory efficacy of standardized Annona muricata (Graviola) leaf extract via activation of mitogen-activated protein kinase pathways in RAW 264.7 macrophages. Evid Based Complement Alternat Med 2016:2905127
Kumari A, Kakkar P (2012) Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci 90:561–570
Laksmitawati DR, Prasanti AP, Larasinta N, Syauta GA, Hilda R, Ramadaniati HU, Widyastuti A, Karami N, Afni M, Rihibiha DD, Kusuma HSW, Widowati W (2016) Anti-inflammatory potential of gandarusa (Gendarussa vulgaris Nees) and soursoup (Annona muricata L) extracts in LPS stimulated-macrophage cell (RAW264.7). J Nat Remed 16:73–81
Lee WM, Seremba E (2009) Drug-induced liver disease. In: Yamada T (ed) Textbook of gastroenterology. Blackwell Publishing, Oxford, pp 2167–2184
Li G, Chen JB, Wang C, Xu Z, Nie H, Qin XY, Chen XM, Gong Q (2013) Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J Gastroenterol 19:7440–7446
Li L, Huang W, Wang S, Sun K, Zhang W, Ding Y, Zhang L, Tumen B, Ji L, Liu C (2018) Astragaloside IV attenuates acetaminophen-induced liver injuries in mice by activating the Nrf2 signaling pathway. Molecules 23. https://doi.org/10.3390/molecules23082032
Lin G, Luo D, Liu J, Wu X, Chen J, Huang Q, Su L, Zeng L, Wang H, Su Z (2018) Hepatoprotective effect of polysaccharides isolated from Dendrobium officinale against acetaminophen-induced liver injury in mice via regulation of the Nrf2-Keap1 signaling pathway. Oxidative Med Cell Longev 2018:6962439
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Moghadamtousi SZ, Kadir HA, Paydar M, Rouhollahi E, Karimian H (2014a) Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-kappaB. BMC Complement Altern Med 14:299
Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Abdulla MA, Kadir HA (2014b) Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther 8:2099–2110
Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA (2015a) Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16:15625–15658
Moghadamtousi SZ, Rouhollahi E, Hajrezaie M, Karimian H, Abdulla MA, Kadir HA (2015b) Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int J Surg 18:110–117
Ning C, Gao X, Wang C, Kong Y, Liu Z, Sun H, Sun P, Huo X, Ma X, Meng Q, Liu K (2018) Ginsenoside Rg1 protects against acetaminophen-induced liver injury via activating Nrf2 signaling pathway in vivo and in vitro. Regul Toxicol Pharmacol 98:58–68
Quilez AM, Montserrat-de la Paz S, De la Puerta R, Fernández-Arche MA, García-Giménez MD (2015) Validation of ethnopharmacological use as anti-inflammatory of a decoction from Annona Muricata leaves. Afr J Tradit Complement Altern Med 12:14–20
Rajasekaran A, Periyasamy M (2012) Hepatoprotective effect of ethanolic extract of Trichosanthes lobata on paracetamol-induced liver toxicity in rats. Chin Med 7:12
Reisman SA, Csanaky IL, Aleksunes LM, Klaassen CD (2009) Altered disposition of acetaminophen in Nrf2-null and Keap1-knockdown mice. Toxicol Sci 109:31–40
Siddiqui RA, Simjee SU, Kabir N, Ateeq M, Shah MR, Hussain SS (2018) N-(2-hydroxyphenyl)acetamide and its gold nanoparticle conjugation prevent glycerol-induced acute kidney injury by attenuating inflammation and oxidative injury in mice. Mol Cell Biochem 450(1–2):43–52
Waters E, Wang JH, Redmond HP, Wu QD, Kay E, Bouchier-Hayes D (2001) Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am J Physiol Gastrointest Liver Physiol 280:G1274–G1279
Wu H, Zhang G, Huang L, Pang H, Zhang N, Chen Y, Wang G (2017) Hepatoprotective effect of polyphenol-enriched fraction from Folium Microcos on oxidative stress and apoptosis in acetaminophen-induced liver injury in mice. Oxidative Med Cell Longev 2017:3631565
Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol 4:131–142
Zamudio-Cuevas Y, Diaz-Sobac R, Vazquez-Luna A, Landa-Solis C, Cruz-Ramos M, Santamaria-Olmedo M, Martinez-Flores K, Fuentes-Gomez AJ, Lopez-Reyes A (2014) The antioxidant activity of soursop decreases the expression of a member of the NADPH oxidase family. Food Funct 5:303–309
Zuckerbraun BS, Billiar TR (2003) Heme oxygenase-1: a cellular Hercules. Hepatology 37:742–744
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted according to the ethical standards approved by the Committee on Research Ethics for Laboratory Animal Care at the Department of Zoology, Faculty of Science, Helwan University (approval no, HU2017/Z/03).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Brakati, A.Y., Fouda, M.S., Tharwat, A.M. et al. The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ Sci Pollut Res 26, 13539–13550 (2019). https://doi.org/10.1007/s11356-019-04935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04935-3