Abstract
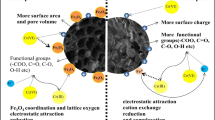
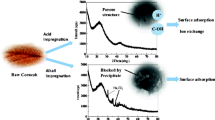
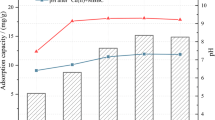
Carbon materials, as effective adsorbents to numerous aqueous cationic contaminants, have been hardly applied to remove anions in wastewater. In this work, different modifying agents were used to modify corncob biochars (CC) and the surface potentials of these modified biochars were determined. Based on the findings, modification principle was determined to reveal the relationship between surface potentials of the biochars and their nitrate adsorption capacities. The surface potential was dominated by the metal cations and multivalent cations led to even positive zeta potential. The formation of metal oxide not only led to the augment in surface area but also increase the surface charge. FeCl3-modified biochar (Fe-CC) with the highest positive surface charge was utilized to remove anions (nitrate) from aqueous solutions. Characterization results confirm that Fe2O3 structure were successfully formed on biochar surface. This led to the formation of iron nitrate hydrate (Fe(NO3)3·9H2O), which enabled higher nitrate adsorption performance than that of pristine biochar. Batch experiments showed that nitrate adsorption on the Fe-CC was stable and almost independent of experimental pH and temperature. Based on the Langmuir model results, the maximum nitrate adsorption capacity of Fe-CC was 32.33 mg/g. Coexisting anions had negative influence on the adsorption performance. Findings of this work suggest that the modified biochar can be used in wastewater treatment to remove anions such as nitrate.

ᅟ









Similar content being viewed by others
References
Calero J, Ontiveros-Ortega A, Aranda V (n.d.) Plaza I Humic acid adsorption and its role in colloidal-scale aggregation determined with the zeta potential, surface free energy and the extended-DLVO theory. Eur J Soil Sci
Choe E, Meer FVD, Rossiter D, Salm CVD, Kim KW (2010) An alternate method for Fourier transform infrared (FTIR) spectroscopic determination of soil nitrate using derivative analysis and sample treatments. Water Air Soil Pollut 206:129–137
Cosimo JID, Dı́Ez VK, Xu M, Iglesia E, CR Apesteguı́A (1998) Structure and surface and catalytic properties of Mg-Al basic oxides ☆. J Catal 178:499–510
Creamer AE, Gao B, Wang SS (2016) Carbon dioxide capture using various metal oxyhydroxide-biochar composites. Chem Eng J 283:826–832
De RG, Lahav M, Me VDB (2014) Pyridine coordination chemistry for molecular assemblies on surfaces. Acc Chem Res 47:3407
Ding Z, Hu X, Wan Y, Wang S, Gao B (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J Ind Eng Chem 33:239–245
Du C, Cui CW, Qiu S, Shi SN, Li A, Ma F (2017) Nitrogen removal and microbial community shift in an aerobic denitrification reactor bioaugmented with a Pseudomonas strain for coal-based ethylene glycol industry wastewater treatment. Environ Sci Pollut Res 24:11435–11445
Eeshwarasinghe D, Loganathan P, Kalaruban M, Sounthararajah DP, Kandasamy J, Vigneswaran S (2018) Removing polycyclic aromatic hydrocarbons from water using granular activated carbon: kinetic and equilibrium adsorption studies. Environ Sci Pollut Res 25:13511–13524
Gao F, Xue YW, Deng PY, Cheng XR, Yang K (2015) Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem Speciat Bioavailab 27:92–97
Hu X, Xue Y, Long L, Zhang K (2018a) Characteristics and batch experiments of acid- and alkali-modified corncob biomass for nitrate removal from aqueous solution. Environ Sci Pollut Res:1–9
Hu XL, Xue YW, Liu LN, Zeng YF, Long L (2018b) Preparation and characterization of Na2S-modified biochar for nickel removal. Environ Sci Pollut Res 25:9887–9895
Jung KW, Lee S, Lee YJ (2017) Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour Technol 245:751–759
Kang J, Duan X, Wang C, Sun H, Tan X, Tade MO, Wang S (2018) Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem Eng J 332:398–408
Liu G, Zhou Y, Liu Z, Zhang J, Tang B, Yang S, Sun C (2016a) Efficient nitrate removal using micro-electrolysis with zero valent iron/activated carbon nanocomposite. J Chem Technol Biotechnol 91:2942–2949
Liu Z, Xue Y, Gao F, Cheng X, Yang K (2016b) Removal of ammonium from aqueous solutions using alkali-modified biochars. Chem Speciat Bioavailab 28:26–32
Long L, Xue Y, Zeng Y, Yang K, Lin C (2017) Synthesis, characterization and mechanism analysis of modified crayfish shell biochar possessed ZnO nanoparticles to remove trichloroacetic acid. J Clean Prod 166:1244–1252
Lu X, Jiang J, Sun K, Zhu G, Lin G (2016) Enhancement of Pb2+ removal by activating carbon spheres/activated carbon composite material with H2O vapor. Colloids Surf A Physicochem Eng Asp 506:637–645
Patwardhan SV, Emami FS, Berry RJ, Jones SE, Naik RR, Deschaume O, Heinz H, Perry CC (2012) Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J Am Chem Soc 134:6244
Qiu Y, Moore S, Hurt R, Külaots I (2017) Influence of external heating rate on the structure and porosity of thermally exfoliated graphite oxide. Carbon 111:651
Sarkar B, Mandal S, Tsang YF, Kumar P, Kim KH, Yong SO (2018) Designer carbon nanotubes for contaminant removal in water and wastewater: a critical review. Sci Total Environ 612:561–581
Sembiring S, Simanjuntak W, Manurung P, Asmi D, Low IM (2014) Synthesis and characterisation of gel-derived mullite precursors from rice husk silica. Ceram Int 40:7067–7072
Sofer Z, Jankovský O, Šimek P, Sedmidubský D, Šturala J, Kosina J, Mikšová R, Macková A, Mikulics M, Pumera M (2015) Insight into the mechanism of the thermal reduction of graphite oxide: deuterium-labeled graphite oxide is the key. ACS Nano 9:5478–5485
Tanboonchuy V, Hsu JC, Grisdanurak N, Liao CH (2011) Impact of selected solution factors on arsenate and arsenite removal by nanoiron particles. Environ Sci Pollut Res 18:857–864
Tytak A, Oleszczuk P, Dobrowolski R (2015) Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ Sci Pollut Res 22:5985–5994
Villegas-Guzman P, Hofer F, Silva-Agredo J, Torres-Palma RA (2017) Role of sulfate, chloride, and nitrate anions on the degradation of fluoroquinolone antibiotics by photoelectro-Fenton. Environ Sci Pollut Res 24:28175–28189
Wan S, Wang S, Li Y, Gao B (2017) Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J Ind Eng Chem 47:246–253
Wang SS, Gao B, Zimmerman AR, Li YC, Ma L, Harris WG, Migliaccio KW (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395
Wang B, S-y L, F-y L, Z-p F (2016) Removal of nitrate from constructed wetland in winter in high-latitude areas with modified hydrophyte biochars. Korean J Chem Eng 34:717–722
Wang S, Li X, Liu Y, Zhang C, Tan X, Zeng G, Song B, Jiang L (2017) Nitrogen-containing amino compounds functionalized graphene oxide: synthesis, characterization and application for the removal of pollutants from wastewater: a review. J Hazard Mater 342:177
Xiao Y, Xue Y, Gao F, Mosa A (2017) Sorption of heavy metal ions onto crayfish shell biochar: effect of pyrolysis temperature, pH and ionic strength. J Taiwan Inst Chem Eng
Xu X, Hu X, Ding Z, Chen Y, Gao B (2017) Waste-art-paper biochar as an effective sorbent for recovery of aqueous Pb(II) into value-added PbO nanoparticles. Chem Eng J 308:863–871
Xue YW, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, Ro KS (2012) Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: batch and column tests. Chem Eng J 200:673–680
Xue L, Gao B, Wan Y, Fang J, Wang S, Li Y, Muñoz-Carpena R, Yang L (2016) High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J Taiwan Inst Chem Eng 63:312–317
Yao Y, Gao B, Inyang M, Zimmerman AR, Cao XD, Pullammanappallil P, Yang LY (2011) Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential. Bioresour Technol 102:6273–6278
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Yu F, Zhou Y, Gao B, Qiao H, Li Y, Wang E, Pang L, Bao C (2016) Effective removal of ionic liquid using modified biochar and its biological effects. J Taiwan Inst Chem Eng 67:318–324
Zhang M, Gao B, Yao Y, Xue YW, Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem Eng J 210:26–32
Zhang M, Gao B, Yao Y, Inyang M (2013) Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 92:1042–1047
Zhang F, Wang X, Xionghui J, Ma L (2016) Efficient arsenate removal by magnetite-modified water hyacinth biochar. Environ Pollut 216:575–583
Acknowledgments
The authors thank the anonymous reviewers for their invaluable insight and helpful suggestions.
Funding
This work was partially supported by the Fundamental Research Funds for the Central Universities (No. 2042016kf0173) and the Wuhan Water Engineering & Technology Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Rights and permissions
About this article
Cite this article
Long, L., Xue, Y., Hu, X. et al. Study on the influence of surface potential on the nitrate adsorption capacity of metal modified biochar. Environ Sci Pollut Res 26, 3065–3074 (2019). https://doi.org/10.1007/s11356-018-3815-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3815-z