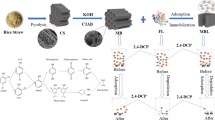
Abstract
Polybrominated diphenyl ethers (PBDEs) are known as ubiquitous pollutants in ecological systems and thus pose a great threat to the health of humans and other organisms due to their bioamplification and bioaccumulation along the food chain. The present study was designed to investigate the biosorption capacity of biochar for the removal of 4-monobromodiphengl ether and its synergistic effect when used as a carrier to immobilize the 4-monobromodiphengl ether-degrading strain Sphingomonas sp. DZ3. The raw biochar material was prepared by pyrolyzing maize straw at 350 °C under oxygen-limited conditions. The maximum biosorption capacity of biochar for 4-bromodiphengl ether was determined to be 50.23 mg/L under an initial concentration of 800 mg/L at pH 7.0 and 40 °C. The data obtained from the biosorption studies were fitted successfully with the pseudo-first-order kinetic and Freundlich isotherm models. The Weber–Morris model analysis indicated that intraparticle diffusion was the limiting step in the biosorption of 4-bromodiphengl ether onto the biosorbent. The values of thermodynamic parameters △G0 were calculated as −24.61 kJ/mol (20 °C), −24.35 kJ/mol (30 °C), and −23.98 kJ/mol (40 °C), △S 0 was −8.45 kJ/mol/K, and △H 0 was 21.36 kJ/mol. The artificial neural network analysis indicated that the initial concentration appeared to be the most influential parameter on the biosorption processes. The removal rate of 4-bromodiphengl ether achieved using the biochar-microorganism system was increased by 63 and 83 % compared with the rates obtained with biochar and the strain individually, respectively. The morphology of the biochar and immobilized strain was determined using a scanning electron microscope, and information of the surface functional groups of biochar was obtained through an infrared spectra study.








Similar content being viewed by others
References
Akar T, Tosun I, Kaynak Z (2009) Assessment of the biosorption characteristics of a macro-fungus for the decolorization of acid red 44 (AR44) dye. J Hazard Mater 171:865–871
Aladağ E, Fil BA, Boncukcuoğlu R, Sözüdoğru O (2014) Adsorption of methyl violet dye, a textile industry effluent onto montmorillonite—batch study. J Dispers Sci Technol 35:1737–1744
Alaee M, Arias P, Sjdin A, Bergman A (2003) An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries, regions and possible modes of release. Environ Int 29:683–689
Alifil B, Ozmetin C (2012) Adsorption of cationic dye from aqueous solution by clay as an adsorbent: thermodynamic and kinetic studies. J Chem Soc Pak 34(4):896–906
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102:716–723
Chen Z, Chen B, Cary T (2012a) Fast and Slow Rates of Naphthalene Sorption to Biochars Produced at Different Temperatures. Environ Sci Technol 46:11104–11111
Chen Z, Chen B, Zhou D, Chen W (2012b) Bisolute Sorption and Thermodynamic Behavior of Organic Pollutants to Biomass-derived Biochars at Two Pyrolytic Temperatures. Environ Sci Technol 46:12476–12483
Chou H, Chang Y, Liao Y, Lin C (2013) Biodegradation of decabromodiphenyl ether (BDE-209) by bacterial mixed cultures in a soil/water system. Int Biodeterior Biodegrad 85:671–682
Cornelissen G, Gustafsson O, Bucheli TD (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39(18):6881–6895
Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A (2008) Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Veterinaria Scandinavica 79:172–183
Devi P, Saroha AK (2014) Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent. Bioresour Technol 169:525–531
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Nat Acad Sci 55:331–333
Freundlich HMF (1906) Über die adsorption in lüsungen. Zeitschrift für Physikalische Chemie 57:385–470
Hashem MA (2007) Adsorption of lead ions from aqueous solution by okra wastes. Int J Theor Phys 2:178–184
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Prot 76:183–191
Ip AWM, Barford JP, McKay G (2009) Reactive black dye adsorption/desorption onto different adsorbents: effect of salt, surface chemistry, pore size and surface area. J Colloid Interface Sci 337:32–38
Jansson B, Andersson R, Asplund L (1993) Chlorinated and brominated persistent organic-compounds in biological samples from the environment. Enveron Toxicol Chem 12:1163–1174
Jia F, Gan J (2014) Comparing black carbon types in sequestering polybrominated diphenyl ethers (PBDEs) in sediments. Environ Pollut 184:131–137
Jiang JJ, Lee CL, Fang MD (2011) Polybrominated diphenyl ethers and polychlorinated biphenyls in sediments of southwest Taiwan: Regional characteristics and potential sources. Mar Pollut Bull 62(4):815–823
Jia X, Liu R, Fan H (2013) Role of sorbent surface functionalities and microporosity in 2, 2’, 4, 4’-tetrabromodiphenyl ether sorption onto biochars. J Environ Sci 25(7):1368–1378
Keiluweit M, Kleber M (2009) Molecular-level interactions in soils and sediments: the role of aromatic π-systems. Environ Sci Technol 43(10):3421–3429
Lagergren S (1898) Zur theorie der sogenannten adsorption gel ster stoffe. Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Olshansky PT, Vetter W (2011) Sorption–desorption behavior of polybrominated diphenyl ethers in soils. Environ Pollut 159:2375–2379
Rivas J, Toral M, Richter P (2012) Sorption of polybrominat ediphenyl ethers in biosolid model amples. J Chil Chem Soc 57(2):1087–1090
Rodenburg LA, Meng Q, Yee D, Ben K (2014) Evidence for photochemical and microbial debromination of polybrominated diphenyl ether flame retardants in San Francisco Bay sediment. Chemosphere 106:36–43
Rosales G, Olguin MT, ColínCruz A, Romero G (2012) Effect of the pH and temperature on the biosorption of lead (II) and cadmium (II) by sodium-modified stalk sponge of Zea mays. Environ Sci Pollut Res 19:177–185
Segev O, Meusel W, Friedenberger M (2009) Aerobic biodegradation of the brominated flame retardants, dibromoneopentyl glycol and tribromoneopentyl alcohol. Biodegradation 20:621–627
Shahryari Z, Sharifi A, Mohebbi A (2013) Artificial neural network (ANN) approach for modeling and formulation of phenol adsorption onto activated carbon. J Eng Thermophys 22:322–336
Singha B, Bar N, Das SK (2014) The use of artificial neural networks (ANN) for modeling of adsorption of Cr (VI) ions. Desalination Water Treat 52:415–425
Weber WJ, Morris JC (1963) Equilibrium and capacities for adsorption on carbon. J Sanit Eng Div 89:31
Wit D (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624
Yang Y, Hunter W, Tao S, Crowley D, Gan J (2009a) Effect of activated carbon on microbial bioavailability of phenanthrene in soils. Environ Toxicol Chem 28:2283–2288
Yang Y, Hunter W, Tao S, Gan J (2009b) Effects of black carbon on pyrethroid availability in sediment. J Agric Food Chem 57:232–238
Yang Y, Jin D, Wang G, Liu D (2011) Biosorption of Acid Blue 25 by unmodified and CPC-modified biomass of Penicillium YW01: kinetic study, equilibrium isotherm and FTIR analysis. Colloids Surf B: Biointerfaces 88:521–526
Yang H, Chou H, Peng Y (2012) Microbial degradation of 4-monobrominated diphenyl ether with anaerobic sludge. J Hazard Mater 213:341–346
Yang Y, Lin X, Wei B, Zhao Y, Wang J (2014) Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. Int J Environ Sci Technol 11:1093–1100
Zhang W, Chen L, Liu K, Chen L, Lin K (2014) Bioaccumulation of decabromodiphenyl ether (BDE209) in earthworms in the presence of lead (Pb). Chemosphere 106:57–64
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Acknowledgments
This study was supported by the National Natural Science Foundation of China (41271335; 31470191), the Major State Basic Research Development Program of China (973 program) (2015CB150502), the High Technology Research and Development Program of China (863 Program) (2012AA06A203), the National Key Technology R and D Program (2012BAC17B04), the Science and Technology Project of Zhejiang Province (2011C13016; 2013C3303; 2014C33019), and the Environmental Science Project of Zhejiang Province (2012B006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Du, J., Sun, P., Feng, Z. et al. The biosorption capacity of biochar for 4-bromodiphengl ether: study of its kinetics, mechanism, and use as a carrier for immobilized bacteria. Environ Sci Pollut Res 23, 3770–3780 (2016). https://doi.org/10.1007/s11356-015-5619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5619-8