Abstract
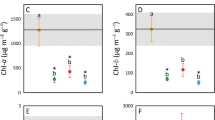
We examined how dominance (% canopy cover) and invasion history of common reed, Phragmites australis, affected benthic macroinvertebrate diversity and density in 8 marshes along Lake Erie’s southern shoreline. We also compared macroinvertebrate densities among patches (0.25 m2) of reed, cattail (Typha spp.), and native flora (e.g., Sagittaria, Sparganium) and epiphytic algal communities on submerged stems of reed and cattail. Narrow-leaf cattail (T. angustifolia) is also a common invasive plant to these wetlands, but does not greatly change plant community composition or ecosystem conditions like reed. Macroinvertebrate diversity (Shannon–Weaver H′) was positively related to reed cover and was highest (4.6) in two marshes with ~35- and 5-year invasion histories. Shading from high reed cover increased H′-diversity, in part, by reducing the abundance of floating duckweed, which harbored many Hyalella azteca amphipods. Percent Ephemeroptera, Odonata, and Trichoptera was low to moderate across marshes, regardless of reed cover and invasion history. Macroinvertebrate density was not affected by reed cover or average plant stem density, and did not differ among plant types. However, epiphyton densities and % diatoms were greater on reed than on cattail, suggesting reed provides a better feeding habitat for microalgal grazers than Typha. Abundance rankings of common species in these diatom-dominated communities were also typically dissimilar between these plant types. Although % grazers was unrelated to epiphyton densities and % diatoms, grazer identity (snails) differed between natural and diked marshes, which had different microalgal food supplies. Our findings suggest that Phragmites does not necessarily adversely affect macroinvertebrate community structure and diversity and that invasion history alone has little effect on the H′-diversity–reed dominance relationship.





Similar content being viewed by others
References
Able KW, Hagan SM (2000) Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: response of fishes and decapod crustaceans. Estuaries 23:633–646
Able KW, Hagan SM (2003) Impact of common reed, Phragmites australis, on essential fish habitat: influence on reproduction, embryological development, and larval abundance of mummichog (Fundulus heteroclitus). Estuaries 26:40–50
Amsberry LM, Baker A, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118
Angradi TR, Hagan SM, Able KW (2001) Vegetation type and the intertidal macroinvertebrate fauna of a brackish marsh: Phragmites vs. Spartina. Wetlands 21:75–92
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC
Armstrong J, Afreen-Zobayed F, Armstrong W (1996) Phragmites die-back: sulphide- and acetic acid-induced bud and root death, lignifications, and blockages with aeration and vascular systems. New Phytol 134:601–614
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19:194–208
Bernstein NP (1981) Vegetational history of Mentor Marsh. J Ohio Acad Sci 81:105–108
Bertness MD (1991) Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72:125–137
Borchardt MA (1996) Nutrients. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, San Diego, pp 184–227
Brigham AR, Brigham WU, Gnilka A (1982) Aquatic insects and oligochaetes of North and South Carolina. Midwest aquatic enterprises. Mahomet, IL
Brower JE, Zar JH, von Ende CN (1998) Field and laboratory methods for general ecology, 4th edn. WCB McGraw Hill, Boston, MA
Brown KM (1982) Resource overlap and competition in pond snails: an experimental analysis. Ecology 63:412–422
Carlson ML, Kowalski KP, Wilcox DA (2009) Promoting species establishment in a Phragmites-dominated Great Lakes coastal wetland. Nat Areas J 29:263–280
Chilton EW (1990) Macroinvertebrate communities associated with three aquatic macrophytes (Ceratophyllum demersum, Myriophyllum spicatum, and Vallisneria americana) in Lake Onalaska, Wisconsin. J Freshw Ecol 5:455–466
Cooper MJ, Uzarski DG, Burton TM (2007) Macroinvertebrate community composition in relation to anthropogenic disturbance, vegetation, and organic sediment depth in four Lake Michigan drowned river-mouth wetlands. Wetlands 27:894–903
Daubenmire R (1968) Plant communities: a textbook of plant synecology. Harper and Row Publishers, New York, p 300
de Szalay FA, Cassidy W (2001) Effects of muskrat (Ondatra zibethicus) lodge construction on invertebrate communities in a Great Lakes coastal wetland. Am Midl Nat 146:300–310
Dvorak J, Best PH (1982) Macro-invertebrate communities associated with macrophytes of Lake Vechten: structural and functional relationships. Hydrobiologia 95:115–126
Fell PE, Weissbach SP, Jones DA, Fallon MA, Zeppieri JA, Faison EK, Lennon KA, Newberry KJ, Reddington LK (1998) Does invasion of oligohaline tidal marshes by reed grass, Phragmites australis (Cav.) Trin. ex. Steud., affect the availability of prey resources for the mummichog, Fundulus heteroclitus L.? J Exp Mar Biol Ecol 222:59–77
Fell PE, Warren RS, Light JK, Rawson L Jr, Fairley SM (2003) Comparison of fish and macorinvertebrate use of Typha angustifolia, Phragmites australis, and treated Phragmites marshes along the lower Connecticut River. Estuaries 26:534–551
Findlay S, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625
Fineran SA (2003) Assessing spatial and temporal vegetative dynamics at Mentor Marsh, 1796 to 2000 AD. PhD dissertation. Ohio State University, Columbus, OH
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755
Gallardo MT, Martin BB, Martin DF (1998) Inhibition of water fern Salvinia minima by cattail (Typha domingensis) extracts and by 2-chlorophenol and salicylaldehyde. J Chem Ecol 24:1483–1490
Gallardo MT, Ascher JR, Collier MJ, Martin BB, Martin DF (1999) Effect of cattail (Typha domingensis) extracts, leachates, and selected phenolic compounds on rates of oxygen production by Salvinia (Salvinia minima). J Aquat Plant Manag 37:80–82
Graca MAS, Cressa C, Gessner MO, Feio MJ, Callies KA, Barrios C (2001) Food quality, feeding preferences, survival and growth of shredders from temperate and tropical streams. Freshw Biol 46:947–957
Güsewell S, Edwards P (1999) Shading by Phragmites australis: a threat for species-rich fen meadows? Appl Veg Sci 2:61–70
Hann BJ (1995) Invertebrate associations with submersed aquatic plants in a praire wetland. University of Manitoba Field Station Delta Marsh, Annual Report 30:78–84
Hanson SR, Osgood DT, Yozzo DJ (2002) Nekton use of a Phragmites australis marsh on the Hudson river, New York, USA. Wetlands 22:326–337
Herdendorf CE, Klarer DM, Herdendorf RC (2004) The ecology of old woman Creek, Ohio: an estuarine and watershed profile. Ohio Department of Natural Resources, Division of Natural Areas and Preserves, Columbus
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol: Annu Rev 35:163–220
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasser flora von Mitteleuropa, Band 2/1. Gustav Fischer Verlag, Stuttgart, New York, pp 1–876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/2. VEB Gustav Fischer Verlag, Jena, pp 1–610
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fischer Verlag, Stuttgart, pp 1–598
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil 1–4. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa, Band 2/4. Gustav Fischer Verlag, Stuttgart, pp 1–437
Kulesza AE, Holomuzki JR (2006) Amphipod performance responses to decaying leaf litter of Phragmites australis and Typha angustifolia from a Lake Erie coastal marsh. Wetlands 26:1079–1088
Kulesza AE, Holomuzki JR, Klarer DM (2008) Benthic community structure in stands of Typha angustifolia and herbicide-treated and untreated Phragmites australis. Wetlands 28:40–56
Kvĕt J, Westlake DF (1998) Primary production in wetlands. In: Westlake DF, Kvĕt J, Szczepański A (eds) The production ecology of wetlands. Cambridge University Press, Cambridge, pp 78–268
Lamberti GA, Moore JW (1984) Aquatic insects as primary consumers. In: Resh VH, Rosenberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 164–195
Levin LA, Talley TS (2000) Influences of vegetation and abiotic environmental factors on salt marsh benthos. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer, Amsterdam, pp 661–708
Lodge DM, Brown KM, Klosiewski SP, Stein RA, Covich AP, Leathers BK, Bronmark C (1987) Distribution of freshwater snails: spatial scale and the relative importance of physiochemical and biotic factors. Am Malacol Bull 5:73–84
Marks M, Lapin B, Randall J (1993) Element stewardship abstract for Phragmites australis. Nature Conservancy, Arlington
Marks M, Lapin B, Randall J (1994) Phragmites australis (P. communis): threats, management, and monitoring. Nat Areas J 14:285–294
McClary M Jr (2004) Spartina alterniflora and Phragmites australis as habitat for the ribbed mussel, Geukensia demissa, in Saw Mill Creek of New Jersey’s Hackensack Meadowlands. Urban Habitats 2:83–90
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America, 3rd edn. Kendall/Hunt Publishing, Dubuque
Meyer SW (2003) Comparative use of Phragmites australis and other habitats by birds, amphibians, and small mammals at Long Point, Ontario. MS thesis, University of Western Ontario, London, Ontario
Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S (2000) A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecol Manage 8:89–103
Morang A, Chader S (2005) Geology and historical evolution of Sheldon Marsh Nature Preserve, Lake Erie, Ohio. US Army Corps of Engineers, Engineer Research and Development Center, Coastal and Hydraulics Laboratory, Vicksburg
Orson RA, Niering WA, Warren RS (1987) The development of a New England river valley tidal marsh. Estuaries 10:20–27
Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ Jr (1990) Freshwater macroinvertebrates of northeastern North America. Cornell University Press, Ithaca
Polunin NVC (1982) Processes contributing to the decay of reed (Phragmites australis) litter in fresh water. Arch Hydrobiol 94:182–209
Posey MA, Alphin TD, Meyer DL, Johnson JM (2003) Benthic communities of common reed Phragmites australis and marsh cordgrass Spartina alterniflora marshes in Chesapeake Bay. Mar Ecol Prog Ser 261:51–61
Raichel DL, Able KW, Hartman JM (2003) The influence of Phragmites (Common Reed) on the distribution, abundance, and potential prey of a resident marsh fish in the Hackensack Meadowlands, New Jersey. Estuaries 26:511–521
Reed PB (1988) National list of plant species that occur in wetlands: Northeast (Region 1). United States Fish and Wildlife Service, Washington, DC, USA. Biological Report 88(26.1)
Robertson TL, Weiss JS (2005) A comparison of epifaunal communities associated with the stems of salt marsh grasses Phragmites australis and Spartina alterniflora. Wetlands 25:1–7
Rooth JE, Stevenson JC (2000) Sediment deposition patterns in Phragmites australis communities: implications for coastal areas threatened by rising sea-level. Wetlands Ecol Manage 8:173–183
Rooth JE, Stevenson JC, Cornwell JC (2003) Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries 26:475–483
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Shih JG, Finkelstein SA (2008) Range dynamics and invasive tendencies in Typha latifolia and Typha angustifolia in Eastern North America derived from herbarium and pollen records. Wetlands 28:1–16
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman and Company, New York
Stewart TW, Downing JA (2008) Macroinvertebrate communities and environmental conditions in recently constructed wetlands. Wetlands 28:141–150
Talley TS, Levin LA (2001) Modification of sediments and macrofauna by an invasive marsh plant. Biol Invasions 3:51–68
Tangen BA, Butler MG, Ell MJ (2003) Weak correspondence between macroinvertebrate assemblages and land use in praire pothole region wetlands, USA. Wetlands 23:104–115
Templer P, Findlay S, Wigand C (1998) Sediment chemistry associated with native and non-native emergent macrophytes of a Hudson River marsh ecosystem. Wetlands 18:70–78
Trexel-Kroll D (2002) Succession of Floating-leaf to emergent plant communities following reduced water levels in Old Woman Creek Estuary. M.Sc. Thesis, Miami University, Oxford, OH, USA
Turner AM, Turner RR, Ray SR (2007) Competition and intraguild egg predation among freshwater snails:re-examining the mechanism of interspecific competition. Oikos 116:1895–1903
USEPA (2002) Methods for evaluating wetland condition: #9 developing an invertebrate index of biological integrity for wetlands. United States Environmental Protection Agency, Office of Water, Washington, DC, USA, EPA-822-R-02-019
Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA (2001) Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries 24:90–107
Wehr JD, Sheath RG (2003) Freshwater algae of North America: ecology and classification. Academic Press, NY
Whipple J (1999) Geological and environmental assessment of Mentor Marsh, Ohio. M.Sc. Thesis, University of Akron, Akron, OH, USA
Whitcraft CR, Levin LA (2007) Regulation of benthic algal and animal communities by salt marsh plants: impact of shading. Ecology 88:904–917
Whyte RS, Franco DA, Klarer DM (2003) The aquatic vegetation of the Old Woman Creek National Estuarine Research Reserve (Huron, Ohio): a Lake Erie coastal wetland. Mich Bot 42:63–84
Whyte RS, Holomuzki JR, Klarer DM (2009) Wetland plant and macroinvertebrate recovery in Phragmites australis-dominated stands after herbicide (Habitat®) treatment). Verh Int Ver Theor Angew Limnol 30:725–730
Wilcox KL, Petrie SA, Maynard LA, Meyer SW (2003) Historical distribution and abundance of Phragmites australis at Long Point, Lake Erie, Ontario. J Great Lakes Res 29:664–680
Wilkinson L (2000) SYSTAT 9. SPSS, Chicago
Acknowledgments
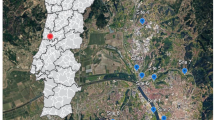
We thank Erie Metro Parks, John McFadden, Doug Brewer, Ron Huffman, Frank Lopez, and Charlotte McCurdy for access to Dupont, Sheldon, Darby, Magee and Metzger, OWC, and Mentor marshes, respectively, Steve Barry for his ArcView expertise in Fig. 1, Grace Kilbane for macroinvertebrate identifications, and Robert Whyte for sharing his historical knowledge of macrophytes in some of these wetlands. Constructive comments by Ferenc de Szalay, Ken Krieger, and an anonymous reviewer significantly improved the manuscript. Funding was provided by a grant to JRH from the National Oceanic and Atmospheric Administration, administered by the Ohio Division of Natural Areas and Preserves through the Ohio Coastal Management Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holomuzki, J.R., Klarer, D.M. Invasive reed effects on benthic community structure in Lake Erie coastal marshes. Wetlands Ecol Manage 18, 219–231 (2010). https://doi.org/10.1007/s11273-009-9161-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-009-9161-7