Abstract
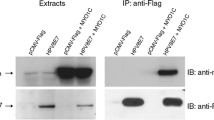
Human papillomaviruses (HPVs) of genus betapapillomavirus (betaHPV) are implicated in skin carcinogenesis, but their exact role in keratinocyte transformation is poorly understood. We show an interaction of HPV5 and HPV8 oncoproteins E6 and E7 with the nuclear mitotic apparatus protein 1 (NuMA). Binding of E6 or E7 to NuMA induces little aneuploidy, cell cycle alterations, or aberrant centrosomes. Intracellular localization of NuMA is not altered by E6 and E7 expression in 2D cultures. However, the localization profile is predominantly cytoplasmic in 3D organotypic skin models. Both viral proteins colocalize with NuMA in interphase cells, while only E7 colocalizes with NuMA in mitotic cells. Intriguingly, a small subset of cells shows E7 at only one spindle pole, whereas NuMA is present at both poles. This dissimilar distribution of E7 at the spindle poles may alter cell differentiation, which may in turn be relevant for betaHPV-induced skin carcinogenesis.




Similar content being viewed by others
References
Howley PM, Pfister HJ (2015) Beta genus papillomaviruses and skin cancer. Virology 479–480:290–296. https://doi.org/10.1016/j.virol.2015.02.004
Smola S (2014) Human papillomaviruses and skin cancer. Adv Exp Med Biol 810:192–207
Tommasino M (2017) The biology of beta human papillomaviruses. Virus Res 231:128–138. https://doi.org/10.1016/j.virusres.2016.11.013
Bouwes Bavinck JN, Feltkamp MCW, Green AC, Fiocco M, Euvrard S, Harwood CA, Nasir S, Thomson J, Proby CM, Naldi L, Diphoorn JCD, Venturuzzo A, Tessari G, Nindl I, Sampogna F, Abeni D, Neale RE, Goeman JJ, Quint KD, Halk AB, Sneek C, Genders RE, de Koning MNC, Quint WGV, Wieland U, Weissenborn S, Waterboer T, Pawlita M, Pfister H, EPI-HPV-VU-CA Group (2018) Human papillomavirus and posttransplantation cutaneous squamous cell carcinoma: a multicenter, prospective cohort study. Am J Transplant 18(5):1220–1230. https://doi.org/10.1111/ajt.14537
Weissenborn SJ, Nindl I, Purdie K, Harwood C, Proby C, Breuer J, Majewski S, Pfister H, Wieland U (2005) Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Investig Dermatol 125(1):93–97
Hasche D, Vinzon SE, Rosl F (2018) Cutaneous papillomaviruses and non-melanoma skin cancer: causal agents or innocent bystanders? Front Microbiol 9:874. https://doi.org/10.3389/fmicb.2018.00874
Quint KD, Genders RE, de Koning MN, Borgogna C, Gariglio M, Bouwes Bavinck JN, Doorbar J, Feltkamp MC (2015) Human beta-papillomavirus infection and keratinocyte carcinomas. J Pathol 235(2):342–354. https://doi.org/10.1002/path.4425
Schaper ID, Marcuzzi GP, Weissenborn SJ, Kasper HU, Dries V, Smyth N, Fuchs P, Pfister H (2005) Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res 65(4):1394–1400
Hufbauer M, Lazic D, Akgül B, Brandsma JL, Pfister H, Weissenborn SJ (2010) Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology 403(2):128–136. https://doi.org/10.1016/j.virol.2010.04.013
Westphal K, Akgül B, Storey A, Nindl I (2009) Cutaneous human papillomavirus E7 type-specific effects on differentiation and proliferation of organotypic skin cultures. Cell Oncol 31(3):213–226. https://doi.org/10.3233/clo-2009-0476
Schmitt A, Harry JB, Rapp B, Wettstein FO, Iftner T (1994) Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J Virol 68(11):7051–7059
Akgül B, Ghali L, Davies D, Pfister H, Leigh IM, Storey A (2007) HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp Dermatol 16(7):590–599. https://doi.org/10.1111/j.1600-0625.2007.00569.x
Akgül B, Garcia-Escudero R, Ghali L, Pfister HJ, Fuchs PG, Navsaria H, Storey A (2005) The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res 65(6):2216–2223
Heuser S, Hufbauer M, Steiger J, Marshall J, Sterner-Kock A, Mauch C, Zigrino P, Akgül B (2016) The fibronectin/alpha3beta1 integrin axis serves as molecular basis for keratinocyte invasion induced by betaHPV. Oncogene 35(34):4529–4539. https://doi.org/10.1038/onc.2015.512
Nguyen CL, Munger K (2009) Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J Virol 83(4):1700–1707. https://doi.org/10.1128/jvi.01971-08
Kallajoki M, Harborth J, Weber K, Osborn M (1993) Microinjection of a monoclonal antibody against SPN antigen, now identified by peptide sequences as the NuMA protein, induces micronuclei in PtK2 cells. J Cell Sci 104(Pt 1):139–150
Silk AD, Holland AJ, Cleveland DW (2009) Requirements for NuMA in maintenance and establishment of mammalian spindle poles. J Cell Biol 184(5):677–690. https://doi.org/10.1083/jcb.200810091
Iwakiri Y, Kamakura S, Hayase J, Sumimoto H (2013) Interaction of NuMA protein with the kinesin Eg5: its possible role in bipolar spindle assembly and chromosome alignment. Biochem J 451(2):195–204. https://doi.org/10.1042/bj20121447
Maiato H, Pereira AJ (2017) Cell division: NuMA bears the load in the spindle. Curr Biol 27(15):R765–R767. https://doi.org/10.1016/j.cub.2017.06.060
Chandramouly G, Abad PC, Knowles DW, Lelievre SA (2007) The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci 120(Pt 9):1596–1606. https://doi.org/10.1242/jcs.03439
Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437:275–280
Caldeira S, Zehbe I, Accardi R, Malanchi I, Dong W, Giarre M, de Villiers EM, Filotico R, Boukamp P, Tommasino M (2003) The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol 77(3):2195–2206
Oswald E, Reinz E, Voit R, Aubin F, Alonso A, Auvinen E (2017) Human papillomavirus type 8 E7 protein binds nuclear myosin 1c and downregulates the expression of pre-rRNA. Virus Genes 53(6):807–813. https://doi.org/10.1007/s11262-017-1491-6
Mendoza JA, Jacob Y, Cassonnet P, Favre M (2006) Human papillomavirus type 5 E6 oncoprotein represses the transforming growth factor beta signaling pathway by binding to SMAD3. J Virol 80(24):12420–12424. https://doi.org/10.1128/jvi.02576-05
Sagnia B, Ateba Ndongo F, Ndiang Moyo Tetang S, Ndongo Torimiro J, Cairo C, Domkam I, Agbor G, Mve E, Tocke O, Fouda E, Ouwe Missi Oukem-Boyer O, Colizzi V (2011) Reference values of lymphocyte subsets in healthy, HIV-negative children in Cameroon. Clin Vaccine Immunol 18(5):790–795. https://doi.org/10.1128/cvi.00483-10
Heuser S, Hufbauer M, Marx B, Tok A, Majewski S, Pfister H, Akgül B (2016) The levels of epithelial anchor proteins beta-catenin and zona occludens-1 are altered by E7 of human papillomaviruses 5 and 8. J Gen Virol 97(2):463–472. https://doi.org/10.1099/jgv.0.000363
Buitrago-Perez A, Hachimi M, Duenas M, Lloveras B, Santos A, Holguin A, Duarte B, Santiago JL, Akgül B, Rodriguez-Peralto JL, Storey A, Ribas C, Larcher F, del Rio M, Paramio JM, Garcia-Escudero R (2012) A humanized mouse model of HPV-associated pathology driven by E7 expression. PLoS ONE 7(7):e41743. https://doi.org/10.1371/journal.pone.0041743
Sperling T, Oldak M, Walch-Ruckheim B, Wickenhauser C, Doorbar J, Pfister H, Malejczyk M, Majewski S, Keates AC, Smola S (2012) Human papillomavirus type 8 interferes with a novel C/EBPbeta-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog 8(7):e1002833. https://doi.org/10.1371/journal.ppat.1002833
Hufbauer M, Cooke J, van der Horst GT, Pfister H, Storey A, Akgül B (2015) Human papillomavirus mediated inhibition of DNA damage sensing and repair drives skin carcinogenesis. Mol Cancer 14(1):183. https://doi.org/10.1186/s12943-015-0453-7
Duensing S, Munger K (2002) The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res 62(23):7075–7082
Heilman SA, Nordberg JJ, Liu Y, Sluder G, Chen JJ (2009) Abrogation of the postmitotic checkpoint contributes to polyploidization in human papillomavirus E7-expressing cells. J Virol 83(6):2756–2764. https://doi.org/10.1128/jvi.02149-08
Patel D, Incassati A, Wang N, McCance DJ (2004) Human papillomavirus type 16 E6 and E7 cause polyploidy in human keratinocytes and up-regulation of G2-M-phase proteins. Cancer Res 64(4):1299–1306
Thomas JT, Laimins LA (1998) Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J Virol 72(2):1131–1137
Hübbers CU, Akgül B (2015) HPV and cancer of the oral cavity. Virulence 6(3):244–248. https://doi.org/10.1080/21505594.2014.999570
Hasche D, Stephan S, Braspenning-Wesch I, Mikulec J, Niebler M, Grone HJ, Flechtenmacher C, Akgül B, Rösl F, Vinzon SE (2017) The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLoS Pathog 13(11):e1006723. https://doi.org/10.1371/journal.ppat.1006723
Hufbauer M, Akgül B (2017) Molecular mechanisms of human papillomavirus induced skin carcinogenesis. Viruses 9(7):187. https://doi.org/10.3390/v9070187
Viarisio D, Muller-Decker K, Accardi R, Robitaille A, Durst M, Beer K, Jansen L, Flechtenmacher C, Bozza M, Harbottle R, Voegele C, Ardin M, Zavadil J, Caldeira S, Gissmann L, Tommasino M (2018) Beta HPV38 oncoproteins act with a hit-and-run mechanism in ultraviolet radiation-induced skin carcinogenesis in mice. PLoS Pathog 14(1):e1006783. https://doi.org/10.1371/journal.ppat.1006783
Sun QY, Schatten H (2006) Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein. Front Biosci 11:1137–1146
Furuta R, Hirai Y, Katase K, Tate S, Kawaguchi T, Akiyama F, Kato Y, Kumada K, Iwasaka T, Yaegashi N, Kanazawa K, Yoshikawa H, Kitagawa T (2003) Ectopic chromosome around centrosome in metaphase cells as a marker of high-risk human papillomavirus-associated cervical intraepithelial neoplasias. Int J Cancer 106(2):167–171. https://doi.org/10.1002/ijc.11216
Duensing S, Duensing A, Flores ER, Do A, Lambert PF, Munger K (2001) Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J Virol 75(16):7712–7716. https://doi.org/10.1128/jvi.75.16.7712-7716.2001
Yu Y, Munger K (2012) Human papillomavirus type 16 E7 oncoprotein engages but does not abrogate the mitotic spindle assembly checkpoint. Virology 432(1):120–126. https://doi.org/10.1016/j.virol.2012.06.006
Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ (1998) Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA 95(25):14711–14716
Rodriguez-Paredes M, Bormann F, Raddatz G, Gutekunst J, Lucena-Porcel C, Kohler F, Wurzer E, Schmidt K, Gallinat S, Wenck H, Rowert-Huber J, Denisova E, Feuerbach L, Park J, Brors B, Herpel E, Nindl I, Hofmann TG, Winnefeld M, Lyko F (2018) Methylation profiling identifies two subclasses of squamous cell carcinoma related to distinct cells of origin. Nat Commun 9(1):577. https://doi.org/10.1038/s41467-018-03025-1
di Pietro F, Echard A, Morin X (2016) Regulation of mitotic spindle orientation: an integrated view. EMBO Rep 17(8):1106–1130. https://doi.org/10.15252/embr.201642292
Hufbauer M, Biddle A, Borgogna C, Gariglio M, Doorbar J, Storey A, Pfister H, Mackenzie I, Akgül B (2013) Expression of betapapillomavirus oncogenes increases the number of keratinocytes with stem cell-like properties. J Virol 87(22):12158–12165. https://doi.org/10.1128/jvi.01510-13
Acknowledgements
This work was supported by the Canceropole Grand-Est/DKFZ Grant to AA and FA, and by the Finnish Society of Sciences and Letters Grant (EA). MK was supported by the Wilhelm-Sander Stiftung für Krebsforschung (Grant No. 2012.105.3). MH was supported by the Deutsche Krebshilfe (Grant No. 70112727). We thank Dr. Ramon Garcia-Escudero (CIEMAT, Madrid, Spain) for the HPV5 E7 mutant constructs and Professor Slawomir Majewski (Medical University in Warsaw, Poland) for providing EV skin sections.
Author information
Authors and Affiliations
Contributions
EO, MK, FA, and MH performed the experiments. AA and EA conceived and designed the study. AA, BA, and EA analyzed the data and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for the use of skin biopsies of epidermodysplasia verruciformis patients for scientific purposes was obtained from the Ethics Committee of the Medical University of Warsaw.
Informed consent
All authors have reviewed the final version of the manuscript and approve it for publication.
Additional information
Edited by Hartmut Hengel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11262_2019_1685_MOESM1_ESM.pptx
Supplementary Fig. 1. Colocalization of HPV5 E6 or wild-type E7, as well as six HPV5 E7 mutants with NuMA in interphase nuclei. COS-7 cells were transfected with expression constructs for E6, or wild-type of mutant E7 tagged with the AU1 epitope. Fixed cells were stained with anti-AU1 (green) and anti-NuMA (red) antibodies. The images show z-axis projections of 12–20 layers. PDM (pixel density mean) values [=(intensity or red color minus mean intensity of red color) × (intensity or green color minus mean intensity of green color)] of colocalizing pixels at each layer were calculated, and they are shown as z-axis projections in pseudocolor. PDM was calculated using the Intensity Correlation Analysis Plugins of ImageJ. Supplementary material 1 (PPTX 1446 kb)
11262_2019_1685_MOESM2_ESM.pptx
Supplementary Fig. 2. HPV5 or HPV8 E6E7 do not cause enhanced aneuploidy. Nonsynchronized PHK cells were transduced with the pLXSN vector, or constructs expressing E6 and E7 of either HPV5, HPV8 or HPV16, and (A) treated with DMSO only (−Noc) or (B) treated with 100 ng/ml nocodazol for 24 hours (+Noc). The cells were harvested, fixed, stained with propidium iodide, and examined by FACS analysis. Results of one representative experiment is shown. (C) Quantification of DNA contents in cells with or without nocodazole treatment. Results from n = 2 in duplicates are shown. The numbers express percentage of cells of the total cell population with DNA content exceeding 4N (%).Supplementary material 2 (PPTX 90 kb)
11262_2019_1685_MOESM3_ESM.pptx
Supplementary Fig. 3. Cell cycle profile of cells expressing HPV5, HPV8 or HPV16 E6E7. Nonsynchronized PHK cells were transduced with pLXSN vector, or constructs expressing E6 and E7 of either HPV5, HPV8 or HPV16, incubated with 100 µM BrdU for 30 minutes and fixed. DNA was visualized with propidiumiodide and BrdU was stained with a specific antibody, and the cells were analyzed using FACS. (A) Histograms of one representative experiment are shown. (B) Quantification of cell cycle profiles using BrdU and PI staining (n = 2 in duplicates), or using PI stained samples (without BrdU, n = 3 in duplicates). The cell cycle profiles of the latter were analyzed using the Dean-Jett-Fox Model of the Cell Cycle Platform in FlowJo. Supplementary material 3 (PPTX 111 kb)
Rights and permissions
About this article
Cite this article
Oswald, E., Kirschberg, M., Aubin, F. et al. BetaHPV E6 and E7 colocalize with NuMa in dividing keratinocytes. Virus Genes 55, 600–609 (2019). https://doi.org/10.1007/s11262-019-01685-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-019-01685-9