Abstract
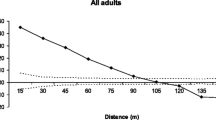
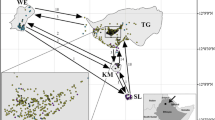
Urban vegetation is an essential requirement in cities for mitigating pollution, heat island effects and providing food and shelter to urban fauna. Efforts to conserve and augment green cover in cities, however, often lack data on the genetic diversity of urban trees, which could be crucial to the success of such programmes. We investigate the population genetics of the cluster fig Ficus racemosa, which occurs naturally in Indian cities and is a keystone species for urban fauna. Genetic analysis of 51 F. racemosa trees in urban Bangalore, India, shows no evidence of inbreeding; the overall inbreeding coefficient (F is ) across 12 microsatellite markers (0.0366) was non-significant with no evidence of heterozygote deficit. Spatial genetic structure (SGS) analysis of 47 trees showed an overall negative relationship between kinship coefficient and spatial distance, with strong SGS at distances <1 km. The absence of heterozygote deficit is likely due to the fig’s obligate mutualistic association with fig wasps which pollinate their flowers even across long distances. However, the strong SGS at short distances could result from clumped seed dispersal close to natal trees. Therefore, the pattern of population genetics for F. racemosa from urban Bangalore likely results from short-distance seed dispersal and long-distance pollen flow. Despite the scattered and fragmented nature of green areas within cities, these gene mobility factors maintain robust population genetics in F. racemosa even at low population densities. The same may not apply for Ficus species that are planted as vegetative cuttings in cities and therefore may have limited genetic diversity.




Similar content being viewed by others
References
Acar C, Acar H, Eroğlu E (2007) Evaluation of ornamental plant resources to urban biodiversity and cultural changing: A case study of residential landscapes in Trabzon city (Turkey). Build Environ 42:218–229
Ahmed S, Compton SG, Butlin RK, Gilmartin PM (2009) Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. Proc Natl Acad Sci U S A 106:20342–20347
Anstett M-C, Hossaert-McKey M, McKey D (1997) Modeling the persistence of small populations of strongly interdependent species: Figs and fig wasps. Conserv Biol 11:204–213
Bain A, Borges RM, Chevallier MH, Vignes H, Kobmoo N, Peng YQ, Cruaud A, Rasplus JY, Kjellberg F, Hossaert-Mckey M (2016) Geographic structuring into vicariant species-pairs in a wide-ranging, high-dispersal plant–insect mutualism: the case of Ficus racemosa and its pollinating wasp s. Evol Ecol 30:663–684
Bartlewicz J, Vandepitte K, Jacquemyn H, Honnay O (2015) Population genetic diversity of the clonal self-incompatible herbaceous plant Linaria vulgaris along an urbanization gradient. Biol J Linn Soc 116:603–613
Berg CC (1989) Classification and distribution of Ficus. Experientia 45:605–611
Borges RM (1993) Figs, Malabar giant squirrels and fruit shortages within two tropical Indian forests. Biotropica 25:183–190
Borges RM (2015) How to be a fig wasp parasite on the fig–fig wasp mutualism. Curr Opin Insect Sci 8:34–40
Borges RM, Ranganathan Y, Krishnan A, Ghara M, Pramanik G (2011) When should fig fruit produce volatiles? Pattern in a ripening process. Acta Oecol 37:611–618
Cannell MG (1999) Environmental impacts of forest monocultures: water use, acidification, wildlife conservation, and carbon storage. New For 17:239262
Carr DE, Eubanks MD (2002) Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae). Evolution 56:22–30
Chen Y, Jiang ZX, Compton SG, Liu M, Chen XY (2011) Genetic diversity and differentiation of the extremely dwarf Ficus tikoua in Southwestern China. Biochem Syst Ecol 39:441–448
Compton SG (2002) Sailing with the wind: Dispersal by small flying insects. In: Bullock JM, Kenward R, Hails RS (eds) Dispersal ecology. Cambridge University Press, Cambridge, pp 113–133
Compton SG, Ellwood MDF, Davis AJ, Welch K (2000) The flight heights of chalcid wasps (Hymenoptera, Chalcidoidea) in a lowland Bornean rain forest: Fig wasps are the high fliers. Biotropica 32:515–522
Cook JM, Rasplus J-Y (2003) Mutualists with attitude: Coevolving fig wasps and figs. Trends Ecol Evol 18:241–248
Corlett RT (2005) Interactions between birds, fruit bats and exotic plants in urban Hong Kong, South China. Urban Ecosyst 8:275–283
Corlett RT (2006) Figs (Ficus, Moraceae) in Urban Hong Kong, South China. Biotropica 38:116–121
Cossalter C, Pye-Smith C (2003) Fast-wood forestry: myths and realities. Center for International Forestry Research (CIFOR), Bogor, Indonesia
Crozier YC, Jia XC, Yao JY, Field AR, Cook JM, Crozier RH (2007) Microsatellite primers for Ficus racemosa and Ficus rubiginosa. Mol Ecol Notes 7:57–59
Davies SJ, Cavers S, Finegan B, White A, Breed MF, Lowe AJ (2015) Pollen flow in fragmented landscapes maintains genetic diversity following stand-replacing disturbance in a neotropical pioneer tree, Vochysia ferruginea Mart. Heredity 115:125–129
Dev SA, Kjellberg F, Hossaert-McKey M, Borges RM (2011) Fine-scale population genetic structure of two dioecious Indian keystone species, Ficus hispida and Ficus exasperata (Moraceae). Biotropica 43(3):309–316
Dew JL, Wright P (1998) Frugivory and seed dispersal by four species of primates in Madagascar’s eastern rain forest. Biotropica 30:425–437
Dunn DW, Yu DW, Ridley J, Cook JM (2008) Longevity, early emergence and body size in a pollinating fig wasp–implications for stability in a fig–pollinator mutualism. J Anim Ecol 77:927–935
Edmondson JL, Stott I, Davies ZG, Gaston KJ, Leake JR (2016) Soil surface temperatures reveal moderation of the urban heat island effect by trees and shrubs. Sci Rep 6. https://doi.org/10.1038/srep33708
Ellegren H (2004) Microsatellites: simple sequences with complex evolution. Nat Rev Genet 5:435–445
Erskine PD, Lamb D, Bristow M (2006) Tree species diversity and ecosystem function: can tropical multi-species plantations generate greater productivity? For Ecol Manag 233:205–210
Escobedo FJ, Kroeger T, Wagner JE (2011) Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ Pollut 159:2078–2087
Frankham R (1995) Inbreeding and extinction: a threshold effect. Conserv Biol 9:792–799
Galil J, Eisikowitch D (1968) On the pollination ecology of Ficus sycomorus in East Africa. Ecology 49:259–269
Ghara M, Borges RM (2010) Comparative life-history traits in a fig wasp community: implications for community structure. Ecol Entomol 35:139–148
Grison-Pigé L, Bessière J-M, Hossaert-McKey M (2002) Specific attraction of fig-pollinating wasps: role of volatile compounds released by tropical figs. Chem Ecol 2:283–295
Hamrick JL, Godt MJW, Murawski DA, Loveless MD (1991) Correlations between species, traits and allozyme diversity: Implications for conservation biology. In: Falk DA and Olsinger KE (eds). Oxford University Press, New York, pp. 75–86
Hardy OJ, Vekemans X (2002) SPAGeDi: A versatile computer program to analyze spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Harrison RD, Rasplus J-Y (2006) Dispersal of fig pollinators in Asian tropical rain forests. J Trop Ecol 22:631–639
Herre EA, Jandér KC, Machado CA (2008) Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. Annu Rev Ecol Evol Syst 39:439–458
Jevanandam N, Goh AG, Corlett RT (2013) Climate warming and the potential extinction of fig wasps, the obligate pollinators of figs. Biol Lett 9. https://doi.org/10.1098/rsbl.2013.0041
Karnosk DF (1979) Dutch elm disease: A review of the history, environmental implications, control, and research needs. Environ Conserv 6:311–322
Kjellberg F, Doumesche B, Bronstein JL (1988) Longevity of a fig wasp (Blastophaga psenes). P K Ned Akad Wetensc 91:117–122
Krishnan A, Borges RM (2014) Parasites exert conflicting selection pressures to affect reproductive asynchrony of their host plant in an obligate pollination mutualism. J Ecol 102:1329–1340
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Loiselle BA, Sork VL, Nason J, Graham C (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425
Lok AFSL, Ang WF, Ng BYQ, Leong TM, Yeo CK, Tan HT (2013) Native fig species as a keystone resource for the Singapore urban environment. Raffles Museum of Biodiversity Research. National University of Singapore, Singapore
Michaloud G, Michaloud-Pelletier S (1987) Ficus hemi-epiphytes (Moraceae) et arbres supports. Biotropica 19:125–136
Muscarella R, Fleming TH (2007) The role of frugivorous bats in tropical forest succession. Biol Rev 82:573–590
Nagamitsu T, Kikuchi S, Hotta M, Kenta T, Hiura T (2014) Effects of population size, forest fragmentation, and urbanization on seed production and gene flow in an endangered maple (Acer miyabei). Am Midl Nat 172:303–316
Nason JD, Hamrick JL (1997) Reproductive and genetic consequences of forest fragmentation: Two case studies of Neotropical canopy trees. J Hered 88:264–276
Nason JD, Herre EA, Hamrick JL (1996) Paternity analysis of the breeding structure of strangler fig populations: Evidence for substantial long-distance wasp dispersal. J Biogeogr 23:501–512
Nason JD, Herre EA, Hamrick JL (1998) The breeding structure of a tropical keystone plant resource. Nature 391:685–687
Nazareno AG, de Carvalho D (2009) What are the reasons for no inbreeding and high genetic diversity of the Neotropical fig tree Ficus arpazusa? Conserv Genet 10:1789–1793
Nazareno AG, Alzate-Marin AL, Pereira RAS (2013) Dioecy, more than monoecy, affects plant spatial genetic structure: the case study of Ficus. Ecol Evol 3:3495–3508
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Noreen AME, Niissalo MA, Lum SKY, Webb EL (2016) Persistence of long-distance, insect-mediated pollen movement for a tropical canopy tree species in remnant forest patches in an urban landscape. Heredity 117:472–480
O'Brien SJ, Evermann JF (1988) Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol Evol 3:25–259
van Oosterhout C, Hutchinson WTF, Willis DPM, Shipley P (2004) MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Pataki DE (2013) City trees: Urban greening needs better data. Nature 502:624–624
Piotto D (2008) A meta-analysis comparing tree growth in monocultures and mixed plantations. For Ecol Manage 255:781–786
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J For 104:118–124
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Ranganathan Y, Borges RM (2009) Predatory and trophobiont-tending ants respond differently to fig and fig wasp volatiles. Anim Behav 77:1539–1545
Ranganathan Y, Ghara M, Borges RM (2010) Temporal associations in fig–wasp–ant interactions: diel and phenological patterns. Entomol Exp Appl 137:50–61
Rousset F, Raymond M (2007) Genepop (v4. 0). Documentation for Windows and Linux. http://kimura.univmontp2.fr/~ rousset/Genepop.htm. 2007
Roy S, Byrne J, Pickering C (2012) A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For Urban Green 11:351–363
Santamour FS Jr (2004) Trees for urban planting: diversity uniformity, and common sense. In: Elevitch CR (ed) The overstory book: Cultivating connections with trees, 2nd edn. Permanent Agricultural Resources, Hawaii, pp 396–399
Shanahan M (2016) Gods, wasps and stranglers. The secret history and redemptive future of fig trees. Chelsea Green Publishing, Vermont
Shanahan M, So S, Compton SG, Corlett R (2001) Fig-eating by vertebrate frugivores: A global review. Biol Rev 76:529–572
Somme L, Moquet L, Quinet M, Vanderplanck M, Michez D, Lognay G, Jacquemart AL (2016) Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst 19:1149–1161
Stagoll K, Lindenmayer DB, Knight E, Fischer J, Manning AD (2012) Large trees are keystone structures in urban parks. Conserv Lett 5:115–122
Sunnucks P (2000) Efficient genetic markers for population biology. Trends Ecol Evol 15:199–203
Tang ZH, Mukherjee A, Sheng LX, Cao M, Liang B, Corlett RT, Zhang SY (2007) Effect of ingestion by two frugivorous bat species on the seed germination of Ficus racemosa and F. hispida (Moraceae). J Trop Ecol 23:125–127
Tso CP (1996) A survey of urban heat island studies in two tropical cities. Atmos Environ 30:507–519
Van Rossum F (2008) Conservation of long-lived perennial forest herbs in an urban context: Primula elatior as study case. Conserv Genet 9:119–128
Wang RW, Yang CY, Zhao GF, Yang JX (2005) Fragmentation effects on diversity of wasp community and its impact on fig/fig wasp interaction in Ficus racemosa L. J Integr Plant Biol 47:20–26
Wang R, Ai B, Gao BQ, Yu S, Li YY, Chen XY (2009) Spatial genetic structure and restricted gene flow in a functionally dioecious fig, Ficus pumila L. var. pumila (Moraceae). Popul Ecol 51:307–315
Wang H, Sork VL, Wu J, Ge J (2010) Effect of patch size and isolation on mating patterns and seed production in an urban population of Chinese pine (Pinus tabulaeformis Carr.) For Ecol Manage 260:965–974
Ware AB, Compton SG (1994) Responses of fig wasps to host plant volatile cues. J Chem Ecol 20:785–802
Wodkiewicz M, Gruszczyńska B (2014) Genetic diversity and spatial genetic structure of Stellaria holostea populations from urban forest islands. Acta Biol Cracov Ser Bot 56:42–53
Wong NH, Yu C (2005) Study of green areas and urban heat island in a tropical city. Habitat Int 29:547–558
Yadav P, Borges RM (2017) Host–parasitoid development and survival strategies in a non-pollinating fig wasp community. Acta Oecol. https://doi.org/10.1016/j.actao.2017.04.001
Yang J, Yu Q, Gong P (2008) Quantifying air pollution removal by green roofs in Chicago. Atmos Environ 42:7266–7273
Yeh FC, Yang RC, Boyle T (1997) POPGENE. A user-friendly shareware for population genetic analysis. Molecular and Biotechnology Center, University of Alberta, Edmonton, Canada
Yu H, Nason JD (2013) Nuclear and chloroplast DNA phylogeography of obligate pollination mutualism and constraints on range expansion in response to climate change. New Phytol 197(1):276–289
Yu H, Nason JD, Ge X, Zeng J (2010) Slatkin’s Paradox: when direct observation and realized gene flow disagree. A case study in Ficus. Mol Ecol 19:4441–4453
Zavodna M, Arens P, Van Dijk PJ, Partomihardjo T, Vosman B, Van Damme JMM (2005) Pollinating fig wasps: genetic consequences of island recolonization. J Evol Biol 18:1234–1243. https://doi.org/10.1111/j.1420-9101.2005.00937.x
Zhou HP, Chen J (2010) Spatial genetic structure in an understorey dioecious fig species: the roles of seed rain, seed and pollen-mediated gene flow, and local selection. J Ecol 98(5):1168–1177
Acknowledgments
The authors thank Chethana S.L. and Santosh Revadi for help with data collection, Yathiraj Ganesh for sample collections and Sunitha Murray for administrative help. The authors also thank Vignesh Venkateswaran, Mahua Ghara, Yuvaraj Ranganathan, Joyshree Chanam, Lakshya Katariya, Pratibha Yadav, Santosh Revadi and Nikita Zachariah for useful comments on the work. This work was supported by the Ministry of Environment, Forests & Climate Change, Department of Biotechnology, Department of Science and Technology (DST-FIST), Government of India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Krishnan, A., Borges, R.M. A fig tree in a concrete jungle: fine-scale population genetic structure of the cluster fig Ficus racemosa in an urban environment. Urban Ecosyst 21, 171–181 (2018). https://doi.org/10.1007/s11252-017-0707-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-017-0707-9