Abstract
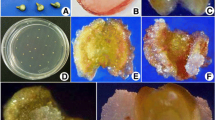
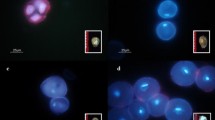
Anther culture techniques were applied to develop a methodology for producing haploid vine cactus plants. Anthers with most microspores in the middle uninucleate developmental stage from the tetraploid species Selenicereus megalanthus and the diploid species Hylocereus polyrhizus and H. undatus were cultured on basal MS medium supplemented with picloram and 6-benzyladenine (BA). H. polyrhizus and H. undatus anthers were also cultured on MS medium containing either thidiazuron (TDZ) or 2,4-dichlorophenoxyacetic acid (2,4-D). Pro-embryo development started after three days of culture. A direct androgenic embryo response was achieved in S. megalanthus with and without picloram/BA, while H. polyrhizus exhibited non-regenerative callus formation. Only a single direct androgenic embryo was obtained in H. polyrhizus (with 0.1 mg/l TDZ). H. undatus required a cold pre-treatment of 4°C for 24 h in 0.3 M D-mannitol to produce a response, but only calluses were obtained and they did not regenerate. S. megalanthus and H. polyrhizus embryos converted into plantlets after transfer to MS medium in the light. Rooted plants were acclimatized successfully, and most plants showed normal phenotypes. Flow cytometry and cytological studies revealed monoploid, haploid, dihaploid, and mixoploid plants. This study showed that androgenesis is strongly species- and culture-medium-dependent, thus revealing new perspectives in the genetics and breeding of vine cactus species. To the best of our knowledge, this is the first report on the production of monoploid, haploid and dihaploid plants in Cactaceae.




Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- MS:
-
Murashige and Skoog
- TDZ:
-
Thidiazuron
References
Barcelo P, Cabrera A, Hagel C, Lorz H (1994) Production of doubled-haploid plants from tritordeum anther culture. Theor Appl Genet 87:741–745. doi:10.1007/BF00222900
Benega R, Isidrón M, Arias E, Cisneros A, Companioni L, Martínez J, Borroto CG (1997) Plant regeneration from pineapple [Ananas comosus (L.) Merr.] ovules. Acta Hortic 425:247–250
Caredda S, Doncoeur C, Devaux P, Sangwan R, Clément SC (2000) Plastid differentiation during androgenesis in albino and non-albino producing cultivars of barley (Hordeum vulgare L.). Sex Plant Reprod 13:95–104. doi:10.1007/s004970000043
Cheema GS, Mehra PN (1981) Anther culture of a cactus Mammillaria elongata var. tenuis (DC) Schumann. Nat Cactus Succulent J 36:8–11
Chen Y, Kenaschuk EO, Procunier JD (1998) Plant regeneration from anther culture in Canadian cultivars of flax (Linum usitatissimum L.). Euphytica 102:183–189. doi:10.1023/A:1018321428210
Daniel G (1993) Anther culture in rye: improved plant regeneration using modified MS-media. Plant Breed 110:259–261. doi:10.1111/j.1439-0523.1993.tb00587.x
Datta SK (2005) Androgenic haploid: factors controlling development and its application in crop improvement. Curr Sci 89:1870–1878
Deepinder G, Gill R, Gosal SS (2006) Role of cysteine in enhancing androgenesis and regeneration of indica rice (Oryza sativa L.). Plant Growth Regul 49:43–47
Doležel J, Binarová P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120. doi:10.1007/BF02907241
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Plant Sci 12:368–375
Germana MA (2006) Doubled haploid production in fruit crops. Plant Cell Tissue Organ Cult 86:131–146. doi:10.1007/s11240-006-9088-0
Gonzalez-Melendi P, Germana MA, Levy Guarda N, Chiancone B, Risueño MC (2005) Correlation of sequential floral and male gametophyte development and preliminary results on anther culture in Opuntia ficus-indica. Acta Physiol Plant 27:687–694. doi:10.1007/s11738-005-0072-9
Han DS, Niimi Y, Nakano M (2000) Long term maintenance of an anther-derived haploid callus line of the Asiatic hybrid lily ‘Connecticut King’. Plant Cell Tissue Organ Cult 61:215–219. doi:10.1023/A:1006425307530
Höfer M, Grafe C (2003) Induction of doubled haploid in sweet cherry (Prunus avium L.). Euphytica 130:191–197. doi:10.1023/A:1022815415604
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119. doi:10.1007/BF01983223
Jacquard C, Asakaviciute R, Hamalian AM, Sangwan RS, Devaux P, Clement C (2006) Barley anther culture: effects of annual cycle and spike position on microspore embryogenesis and albinism. Plant Cell Rep 25:375–381. doi:10.1007/s00299-005-0070-9
Jensen CJ (1974) Chromosome doubling techniques in haploids. In: Kasha KJ (ed) Haploid in higher plants. University of Guelph, Guelph, pp 153–190
Kim M, Jang I, Kim J (2008) Embryogenesis and plant regeneration of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep 27:425–434. doi:10.1007/s00299-007-0442-4
Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo Y, Liu Y (2001) Investigating the hows and whys of DNA endoreduplication. J Exp Bot 52:183–192. doi:10.1093/jexbot/52.355.183
Lichtenzveig J, Abbo S, Nerd A, Tel-Zur N, Mizhrahi Y (2000) Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus. Am J Bot 87:1058–1065. doi:10.2307/2657005
Mendoza MG, Kaeppler HF (2002) Auxin and sugar effects on callus induction and plant regeneration frequencies from mature embryos of wheat (Triticum aestivum L.). In Vitro Cell Dev Biol Plant 38:39–45. doi:10.1079/IVP2001250
Merten S (2003) A review of Hylocereus production in the United States. J PACD 5:98–105
Mizrahi Y, Nerd A (1999) Climbing and columnar cacti. In: Janick J (ed) New arid land fruit crops. Perspective in new crops and new uses. ASHS Press, Alexandria, pp 358–366
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Niimi Y, Han D, Fujisaki M (2001) Production of virus-free plantlets by anther culture of Lilium × ‘Enchantment’. Sci Hortic (Amsterdam) 90:325–334. doi:10.1016/S0304-4238(01)00223-0
Oleszczuk S, Sowa S, Zimny J (2006) Androgenic response to preculture stress in microspore culture of barley. Protoplasma 228:95–100. doi:10.1007/s00709-006-0179-x
Scorza R, Pooler M (1999) Growth and yield of F1 hybrids peaches developed from doubled haploids. HortScience 34:928–931
Soriano M, Cistue L, Castillo AM (2008) Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant Cell Rep 27:805–811. doi:10.1007/s00299-007-0500-y
Tel-Zur N, Abbo S, Bar-Zvi D, Mizhrahi Y (2004) Genetic relationships among Hylocereus and Selenicereus vine cacti (Cactaceae): evidence from hybridization and cytological studies. Ann Bot (Lond) 94:527–534. doi:10.1093/aob/mch183
Tel-Zur N, Abbo S, Mizrahi Y (2005) Cytogenetics of semi-fertile triploid and aneuploid intergeneric vine cacti hybrids. J Hered 6:124–131
Acknowledgments
The authors are grateful to Dr. Manoj Kulkarni, and Aroldo Cisneros and Joseph Mouyal for their valuable assistance in the course of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benega Garcia, R., Schneider, B. & Tel-Zur, N. Androgenesis in the vine cacti Selenicereus and Hylocereus (Cactaceae). Plant Cell Tiss Organ Cult 96, 191–199 (2009). https://doi.org/10.1007/s11240-008-9475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9475-9