Abstract
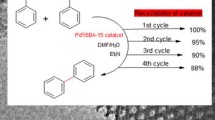
Many efforts have been done to develop new catalysts for organic reactions. In this study, preparation and characterization of palladium immobilized on modified magnetic Fe3O4 nanocatalyst (Fe3O4@SiO2@DPA-Pd) have been reported; dipicolylamine (DPA) groups are used as linkers to fix palladium nanoparticles on silica-coated Fe3O4 nanoparticles without agglomeration. The structure of the nanocatalyst was investigated using scanning electron microscopy, X-ray powder diffraction, Fourier-transform infrared spectroscopy, energy-dispersive X-ray spectroscopy, Brunauer–Emmett–Teller (BET) and thermogravimetry analysis. The data showed that the nanoparticles have spherical morphology with an average size of about 40 nm and 49.16 m2/g BET surface area. The inductively coupled plasma analysis confirmed that the Pd2+ had been successfully loaded on the Fe3O4@SiO2@DPA support with a high amount of 1.03 mmol g−1. The synthesized nanocatalyst was considered for synthesis of biaryls by the Suzuki–Miyaura coupling reaction in water/ethanol as the solvent system and in the absence of toxic phosphine ligand. The reaction conditions were mild, and coupling reaction yields were excellent (68–95%). The synthesis is compatible with the environment. The nanocatalyst can be easily recycled from the reaction mixture using an external magnet and was used at least five times without significant loss of catalytic activity.










Similar content being viewed by others
References
N. Sharma, H. Ojha, A. Bharadwaj, D.P. Pathak, R.K. Sharma, Rsc. Adv. 5, 53381 (2015)
I. IHussain, J. Capricho, M.A. Yawer, Adv. Synth. Catal. 358, 3320 (2016)
Z. Lu, J.B. Jasinski, S. Handa, G.B. Hammond, Org. Biomol. Chem. 16, 2748 (2018)
T.S. Rodrigues, A.G. da Silva, P.H. Camargo, J. Mater. Chem. A. 7, 5857 (2019)
M.B. Gawande, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 3371 (2013)
P. Rai, D. Gupta, Synth. Commun. 51, 3059 (2021)
F. Mousavi, D. Elhamifar, S. Kargar, Surf. Interfaces 25, 101225 (2021)
F. Godarzbod, Z. Mirjafary, H. Saeidian, M. Rouhani, Appl. Organomet. Chem. 35, 6132 (2021)
H. Saeidian, H. Sadighian, M. Arabgari, Z. Mirjafary, S.E. Ayati, E. Najafi, F.M. Moghaddam, Res. Chem. Intermed. 44, 601 (2018)
B. Eshtehardian, M. Rouhani, Z. Mirjafary, J. Iran. Chem. Soc. 17, 469 (2020)
F. Jafari, A. Ghorbani-Choghamarani, N. Hasanzadeh, Res. Chem. Intermed. 47, 1033 (2021)
J. Vela, G.R. Lief, Z. Shen, R.F. Jordan, Organometallics 26, 6624 (2007)
W.F. Maier, K. Stoewe, S. Sieg, Angew. Chem. Int. Ed. 46, 6016 (2007)
F. Ulusal, E. Erünal, B. Güzel, J. Nanopart. Res. 20, 219 (2018)
S. Sain, D. Kishore, S. Jain, V. Sharma, M. Srivastava, N. Sankararamakrishnan, S. Mishra, J. Dwivedi, S.M. Wabaidur, S. Sharma, Inorg. Chem. Commun. 122, 108230 (2020)
A.O. King, N. Yasuda, Organometallics, 7, 205 (2004).
A. Chatterjee, T.R. Ward, Catal. Lett. 146, 820 (2016)
K.C. Nicolaou, P.G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 44, 4442 (2005)
D.E. Jose, U.S. Kanchana, T.V. Mathew, G. Anilkumar, J. Organomet. Chem. 927, 121538 (2020)
A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.M. Basset, V. Polshettiwar, Chem. Soc. Rev. 40, 5181 (2011)
S. Kotha, K. Lahiri, D. Kashinath, Tetrahedron 58, 9633 (2002)
S.E. Hooshmand, B. Heidari, R. Sedghi, R.S. Varma, Green. Chem. 21, 381 (2019)
H. Veisi, S. Najafi, S. Hemmati, Int. J. Biol. Macromol. 113, 186 (2018)
S. Shylesh, L. Wang, W.R. Thiel, Adv. Synth. Catal. 352, 425 (2010)
R. Jahanshahi, B. Akhlaghinia, Catal. Lett. 147, 2640 (2017)
A. Maleki, R. Taheri-Ledari, R. Ghalavand, R. Firouzi-Haji, J. Phys. Chem. Solids 136, 109200 (2020)
P.S. Pharande, G.S. Rashinkar, D.M. Pore, Res. Chem. Intermed. 47, 4457 (2021)
E. Rezapour, M. Jafarpour, A. Rezaeifard, Catal. Lett. 148, 3165 (2018)
M. Pérez-Lorenzo, J. Phys. Chem. Lett. 3, 167 (2012)
T. Baran, M. Nasrollahzadeh, Int. J. Biol. Macromol. 148, 565 (2020)
D. Paul, S. Rudra, P. Rahman, S. Khatua, M. Pradhan, P.N. Chatterjee, J. Organomet. Chem. 871, 96 (2018)
N.Y. Baran, T. Baran, M. Nasrollahzadeh, R.S. Varma, J. Organomet. Chem. 900, 120916 (2019)
I. Sargin, T. Baran, G. Arslan, Sep. Purif. Technol. 247, 116987 (2020)
M. Palaniandavar, R.J. Butcher, A.W. Addison, Inorg. Chem. 35, 467 (1996)
Z. Kowser, H. Tomiyasu, X. Jiang, U. Rayhan, C. Redshaw, T. Yamato, New. J. Chem. 39, 4055 (2015)
M.W. Louie, H.W. Liu, M.H. Lam, T.C. Lau, K.K. Lo, Organometallics 28, 4297 (2009)
M. Çalışkan, T. Baran, M. Nasrollahzadeh, J. Phys. Chem. Solids 152, 109968 (2021)
A. Dadras, M.R. Naimi-Jamal, F.M. Moghaddam, S.E. Ayati, Appl. Organomet. Chem. 32, 3993 (2018)
V. Sadhasivam, B. Sankar, G. Elamathi, M. Mariyappan, A. Siva, Res. Chem. Intermed. 46, 681 (2020)
G. Lv, W. Mai, R. Jin, L. Gao, Synlett 9, 1418 (2008)
F. Heidari, M. Hekmati, H. Veisi, J. Colloid. Interface. Sci. 501, 175 (2017)
M. Ghiaci, M. Zarghani, F. Moeinpour, A. Khojastehnezhad, Appl. Organomet. Chem. 28, 589 (2014)
S. Tahmasebi, J. Mokhtari, M.R. Naimi-Jamal, A. Khosravi, L. Panahi, J. Organomet. Chem. 35, 853 (2017)
N. Miyaura, A. Suzuki, J. Chem. Soc. Chem. Commun. 19, 866 (1979)
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Godarzbod, F., Mirjafary, Z., Saeidian, H. et al. Palladium@silica-coated magnetic nanoparticles as efficient and recyclable catalysts for ligand-free Suzuki–Miyaura coupling reaction under mild conditions. Res Chem Intermed 48, 3685–3699 (2022). https://doi.org/10.1007/s11164-022-04781-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04781-y