Abstract
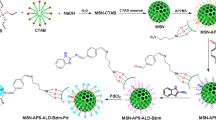
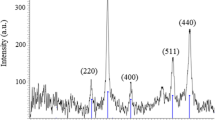
Palladium (Pd) nanoparticles were synthesized using the dendrimer-template method and then immobilized on a silica support (SBA-15). The SBA-15 support material was specifically synthesized with a pore size large enough (d = 6.6 nm) to accommodate the dendrimer generation used (G-5 PAMAM-OH; d = 5.4 nm). The morphology of the Pd-supported catalyst (Pd0/SBA-15) was characterized by scanning electron microscopy coupled to an energy dispersive X-ray spectrometer which confirmed the elemental composition of the catalyst. Transmission electron microscopy confirmed the ordered array of the mesoporous SBA-15 and showed that the Pd NPs were indeed immobilized inside the pores. The catalyst had a pore size of 5.7 nm, a pore volume of 0.78 cm3/g and a surface area of 493 m2/g as determined by surface adsorption/desorption measurements from BET analysis. The prepared Pd0/SBA-15 catalyst was an efficient recyclable heterogeneous catalyst for Suzuki coupling. The catalytic activity was investigated using different experimental conditions such as temperature, catalyst loading, base and solvent variations. The catalyst gave high conversions and turnover frequencies even in aqueous solutions and at fairly low temperatures showing the potential for the green synthesis of important organic molecules through C–C bond formation.
Graphical Abstract










Similar content being viewed by others
References
G. Schmid Clusters and Colloids. From Theory to Applications (VCH, Wienheim, 1994).
Y. S. N. Toshima in A. T. Hubbard (ed.), Encyclopedia of Surface and Colloid Science (Marcel Derker, New York, 2002).
N. Toshima and T. Yonezawa (1998). N. J. Chem. 22, 1179.
N. Miyaura and A. Suzuki (1995). Chem. Rev. 95, 2457.
C. Deraedt, D. Astruc (2013). Accounts of Chemical Research.
C. C. C. JohanssonSeechurn, M. O. Kitching, T. J. Colacot, and V. Snieckus (2012). Angew. Chem. Int. Ed. 51, 5062.
L. Yin and J. Liebscher (2006). Chem. Rev. 107, 133.
C. Tucker and J. de Vries (2002). Top. Catal. 19, 111.
J. Durand, E. Teuma, and M. Gómez (2008). Eur. J. Inorg. Chem. 2008, 3577.
N. Miyaura and A. Suzuki (1979). J. Chem. Soc. Chem. Commun. 19, 866.
N. Miyaura, K. Yamada, and A. Suzuki (1979). Tetrahedron Lett. 20, 3437.
H. Veisi, M. Hamelian, and S. Hemmati (2014). J. Mol. Catal. A 395, 25.
A. Fodor, Z. Hell, and L. Pirault-Roy (2014). Appl. Catal. A 484, 39.
S. Das, S. Bhunia, T. Maity, and S. Koner (2014). J. Mol. Catal. A 394, 188.
A. S. Roy, J. Mondal, B. Banerjee, P. Mondal, A. Bhaumik, and S. M. Islam (2014). Appl. Catal. A 469, 320.
M. M. Heravi, E. Hashemi, Y. S. Beheshtiha, S. Ahmadi, and T. Hosseinnejad (2014). J. Mol. Catal. A 394, 74.
W. R. Reynolds, P. Plucinski, and C. G. Frost (2014). Catal. Sci. Technol. 4, 948.
B. D. Briggs, R. T. Pekarek, and M. R. Knecht (2014). J. Phys. Chem. C 118, 18543.
B. J. Borah, S. J. Borah, K. Saikia, and D. K. Dutta (2014). Appl. Catal. A 469, 350.
A. Alizadeh, M. M. Khodaei, D. Kordestania, and M. Beygzadeh (2013). J. Mol. Catal. A 372, 167.
W. Tang, A. G. Capacci, X. Wei, W. Li, A. White, N. D. Patel, J. Savoie, J. J. Gao, S. Rodriguez, B. Qu, N. Haddad, B. Z. Lu, D. Krishnamurthy, N. K. Yee, and C. H. Senanayake (2010). Angew. Chem. 122, 6015.
D.-H. Lee and M.-J. Jin (2010). Org. Lett. 13, 252.
J.-H. Noh and R. Meijboom (2014). J. Colloid Interface Sci. 415, 57.
I. P. Beletskaya and A. V. Cheprakov (2000). Chem. Rev. 100, 3009.
A. H. M. de Vries, J. M. C. A. Mulders, J. H. M. Mommers, H. J. W. Henderickx, and J. G. de Vries (2003). Org. Lett. 5, 3285.
K. Köhler, R. G. Heidenreich, J. G. E. Krauter, and J. Pietsch (2002). Chem. A Eur. J. 8, 622.
V. Polshettiwar and Á. Molnár (2007). Tetrahedron 63, 6949.
C. Rossy, J. Majimel, M. T. Delapierre, E. Fouquet, and F.-X. Felpin (2014). Appl. Catal. A 482, 157.
M. Nasrollahzadeh, M. Maham, and M. M. Tohidi (2014). J. Mol. Catal. A 391, 83.
R. Franzén (2000). Can. J. Chem. 78, 957.
J. M. Davidson, C. Triggs (1968). J. Chem. Soc. A. 1324–1330.
K. Garves (1970). J. Org. Chem. 35, 3273.
G. Altenhoff, R. Goddard, C. W. Lehmann, and F. Glorius (2003). Angew. Chem. Int. Ed. 42, 3690.
W. A. Herrmann, K. Öfele, S. K. Schneider, E. Herdtweck, and S. D. Hoffmann (2006). Angew. Chem. Int. Ed. 45, 3859.
M. Thimmaiah and S. Fang (2007). Tetrahedron 63, 6879.
D. J. M. Snelders, G. van Koten, and R. J. M. Klein (2009). Gebbink. J. Am. Chem. Soc. 131, 11407.
Y. Uozumi, Y. Matsuura, T. Arakawa, and Y. M. A. Yamada (2009). Angew. Chem. Int. Ed. 48, 2708.
M. Lysén and K. Köhler (2005). Synlett 2005, 1671.
M. Lysén and K. Köhler (2006). Synthesis 2006, 692.
N. Gürbüz, I. Özdemir, T. Seçkin, and B. Çetinkaya (2004). J. Inorg. Organomet. Polym. 14, 149.
D. Zhao, Q. Huo, J. Feng, B. F. Chmelka, and G. D. Stucky (1998). J. Am. Chem. Soc. 120, 6024.
D. Zhao, J. Sun, Q. Li, and G. D. Stucky (2000). Chem. Mater. 12, 275.
E. Vunain, R. Malgas-Enus, K. Jalama, and R. Meijboom (2013). J. Sol-Gel Sci. Technol. 68, 270.
R. W. J. Scott, H. Ye, R. R. Henriquez, and R. M. Crooks (2003). Chem. Mater. 15, 3873.
I. Yuranov, L. Kiwi-Minsker, P. Buffat, and A. Renken (2004). Chem. Mater. 16, 760.
IUPAC (1985). Pure Appl. Chem. 57, 603.
N. Ivashchenko, V. Tertykh, V. Yanishpolskii, S. Khainakov, and A. Dikhtiarenko (2011). Materialwiss. Werkstofftech. 42, 64.
Y. Jiang and Q. Gao (2005). J. Am. Chem. Soc. 128, 716.
S. Jana, S. Haldar, and S. Koner (2009). Tetrahedron Lett. 50, 4820.
S. Bhunia, R. Sen, and S. Koner (2010). Inorg. Chim. Acta 363, 3993.
J. Guerra and M. A. Herrero (2010). Nanoscale 2, 1390.
N. T. S. Phan, M. Van Der Sluys, and C. W. Jones (2006). Adv. Synth. Catal. 348, 609.
Acknowledgments
This work is based on the research supported in part by the National Research Foundation of South Africa (Grant specific unique reference number (UID) 85386). We would also like to thank the University of Johannesburg for funding, Dr. Meyer and Mr. Harris of Shimadzu South Africa for the use of their instruments, Mrs. Tutuzwa, the HRTEM operator at CSIR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ncube, P., Hlabathe, T. & Meijboom, R. Palladium Nanoparticles Supported on Mesoporous Silica as Efficient and Recyclable Heterogenous Nanocatalysts for the Suzuki C–C Coupling Reaction. J Clust Sci 26, 1873–1888 (2015). https://doi.org/10.1007/s10876-015-0885-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0885-7