Abstract
The prefrontal cortex is appreciated as a key neurobiological player in human eating behavior. A special focus is herein dedicated to the dorsolateral prefrontal cortex (DLPFC), which is critically involved in executive function such as cognitive control over eating. Persons with obesity display hypoactivity in this brain area, which is linked to overconsumption and food craving. Contrary to that, higher activity in the DLPFC is associated with successful weight-loss and weight-maintenance. Transcranial direct current stimulation (tDCS) is a non-invasive neurostimulation tool used to enhance self-control and inhibitory control. The number of studies using tDCS to influence eating behavior rapidly increased in the last years. However, the effectiveness of tDCS is still unclear, as studies show mixed results and individual differences were shown to be an important factor in the effectiveness of non-invasive brain stimulation. Here, we describe the current state of research of human studies using tDCS to influence food intake, food craving, subjective feeling of hunger and body weight. Excitatory stimulation of the right DLPFC seems most promising to reduce food cravings to highly palatable food, while other studies provide evidence that stimulating the left DLPFC shows promising effects on weight loss and weight maintenance, especially in multisession approaches. Overall, the reported findings are heterogeneous pointing to large interindividual differences in tDCS responsiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Obesity, one of the most serious public health problems, has reached epidemic proportions [1,2,3]. The WHO estimates that the prevalence of overweight has tripled since 1975, leading to more than 1.9 billion overweight and 650 million obese adult people [4]. Overweight and obesity are associated with a variety of diseases such as several cancer types, dementia, depression, cardiovascular disease [5,6,7] and play a crucial role in the development of type 2 diabetes (T2D) and insulin resistance (IR) [8]. Although obesity and overweight is most commonly caused by a long-term energy imbalance [6], the etiology is multifactorial, including genetic, social, economic, environmental, physiologic and psychological factors [9]. Broadly speaking, the pathophysiology of obesity is complex and still not fully understood. Beyond basic homeostatic mechanisms, the regulation of food intake in humans is established by a core brain network of cognitive control and reward processing pathways (for reviews see [10,11,12,13]). In recent years, modern neuroimaging methods, such as functional magnetic resonance imaging (fMRI), have provided new means to investigate key neurobiological determinants of human eating behavior and offer the opportunity to identify novel target structures for interventions. Accordingly, neuroimaging studies suggest that individuals affected by overweight and obesity show dysregulation of the mesolimbic reward and prefrontal cortex (PFC) cognitive control system (for recent review [13]). In the recent years, new treatment modalities have been explored to facilitate behavioral changes that enable successful weight loss. Non-invasive brain stimulation (NIBS) techniques such as transcranial direct current stimulation (tDCS) represent novel tools able to influence neuronal activity [14,15,16,17,18,19,20]. Yet, it is still unclear if stimulating specific brain areas, linked to overconsumption, improves food related outcomes on the behavioral level. Despite the growing interest in tDCS as an intervention tool, effects are inconsistent making previous results difficult to replicate [21]. Here we review tDCS studies targeting PFC function to influence food craving and food intake with the aim to broaden our understanding of the underlying neurophysiological mechanisms and modulators responsible for the effect of tDCS on eating behavior. The dorsolateral prefrontal cortex (DLPFC) is a key player in dietary self-control and most commonly targeted by tDCS studies on eating behavior. Therefore, this review will specifically focus on tDCS of the DLPFC.
2 DLPFC and its role in eating behavior regulation
The DLPFC is most commonly associated with executive functions, such as working memory, decision-making, problem solving, cognitive control, self-control and response inhibition [22,23,24,25,26]. Naturally, these cognitive functions, due to their complexity, depend on a large distributed brain network. Neuroimaging research showed that various frontal brain regions besides the DLPFC are linked to general cognitive control, including the anterior cingulate cortex [27], the ventrolateral prefrontal cortex [28], the orbitofronal cortex [29], the medial PFC [30] and the inferior frontal gyrus (IFG) [31]. These regions are highly functionally coupled with the DLPFC and most likely act in concert to sustain complex cognitive functions. In the context of eating behavior, neuroimaging evidence displays that DLPFC activity and functional coupling to the ventromedial PFC promote healthy food choices and successful dietary self-control [10, 25, 32]. DLPFC together with IFG activity are vital for the successful suppression of food craving and the motivation to eat [33,34,35,36]. There is some evidence supporting a left–right dichotomy showing that the right PFC to be more involved in inhibitory control and the left PFC in decision making processes as self-control abilities [10] Moreover, the PFC is responsive to a meal, postprandial hormones and to the taste and sight of food [37,38,39,40,41,42,43].
Persons with obesity fail to recruit left DLPFC activity particularly in response to food images [44] and to a meal [37, 45]. Moreover, obese patients with binge-eating disorder (BED) show an attenuated activation of the DLPFC, primarily in the right hemisphere, in response to a food-related response-inhibition task [46]. On the behavioral level, lower inhibitory capacity is linked to a higher Body-Mass-Index (BMI) [47, 48] and higher palatable food consumption [49]. Hence, cognitive control and its underlying neurobiological regulations is a relevant target to improve dysregulated eating and metabolic health.
Indeed, increased activity in the DLPFC and higher inhibitory control is related to successful self-control of food consumption. For instance, higher activation of the right DLPFC in response to high-calorie food images correlated with a subsequent reduction in ad libitum energy intake [50]. Shifting an individual’s attention to healthy eating increased left DLPFC activity [32, 51] and the capacity of activation in the DLPFC seems to be a predictor for weight loss success [52]. In this context, Weygandt et al. [53, 54] reported in two fMRI studies that higher activity in the DLPFC during a food-related decision making task is associated with the success of weight-loss and weight maintenance. Furthermore, adults with higher activation in the DLPFC while resisting food craving displayed better weight loss success following bariatric surgery [55]. In accordance, greater postprandial activation in the left DLPFC was reported in lean and post-obese women who successfully lost weight compared to obese women [45]. Besides being a significant predictor for weight loss success, it has been shown that it is possible to increase DLPFC activity using neurofeedback. A single training session was sufficient for overweight and obese adults to up-regulate their left DLPFC activity [56] as well as functional connectivity to the ventromedial PFC [57].
Taken together, the results suggest that the DLPFC and its functional connections to other frontal regions are vital for successful dietary self-control making this frontal network a prime target for the treatment of obesity. However, to date, it is not clear whether the right or left DLPFC contributes more significantly to dysregulated eating behavior and current literature hold evidence for both [10]. Even though, it is still not clear if failure to appropriately activate the DLPFC is a cause or consequence of obesity, overall, neuroimaging and behavioral data suggest that an increase in the activity of the DLPFC might be effective to lose and maintain body weight.
3 Transcranial direct current stimulation (tDCS)
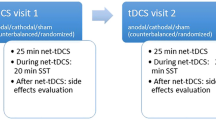
A growing number of studies in recent years have taken the approach of directly manipulating DLPFC activity using NIBS techniques such as repetitive transcranial magnetic stimulation (rTMS) and tDCS. Both approaches are able to modify cortical excitability in the brain [14,15,16,17,18,19,20]. Unlike rTMS, tDCS is less costly and easier to use [58,59,60,61] but cannot trigger an action potential [17]. The technique of tDCS relies on the application of a weak and constant direct current (DC) of mostly 1–2 mA for a duration of approximately 20 min, producing a weak electric field [14, 62, 63]. Usually conventional (i.e. traditional) tDCS deliver DC from a device using two large sponge electrodes [64]. However, a more recent tDCS design called high-definition (HD) uses multiple smaller electrodes [64], increasing focality compared to conventional tDCS [65,66,67]. The electrode formation mostly applied for HD-tDCS is the so-called 4 × 1 ring-configuration. The active electrode is placed over the target area while the four return electrodes are placed around the target, building a ring around the inner electrode [66, 68]. Figure 1 displays these two common tDCS technologies.
Finite element models of transcranial direct current stimulation (tDCS) montages aimed at targeting the dorsolateral prefrontal cortex (DLPFC). (A) The high-definition (HD) tDCS montages is displayed as a 4 × 1 ring montage. (B) The conventional tDCS montage shows two 5 × 7 cm sponge electrodes used in traditional tDCS. Electrode positions are based on the 10–20 international system. Conventional tDCS produces a wide-spread electric-field distribution compared to HD-tDCS which shows a higher focality of the target stimulation [65,66,67]. Figure is adapted from [86]
The underlying principle of action in tDCS is based on a subthreshold modulation of neuronal membrane potentials, leading to an alteration of the cortical excitability [69]. Nitsche and Paulus [14] demonstrated that this effect is polarity dependent with surface anodal tDCS resulting in an increase whilst suface cathodal stimulation showing a decrease of cortical excitability, also referred to as excitatory and inhibitory stimulation, respectively. However, this simple dichotomy of anodal-excitation and cathodal-inhibition (AeCi) is not apparent in all tDCS studies, and especially cathodal stimulation of higher intensities can produce excitatory effects; Batsikadze et al. [70] showed that the application of a 20-min cathodal tDCS, targeting the left primary motor cortex (M1), resulted in an enhancement of cortical excitability when using 2 mA current, while lower electric current (1 mA) decreased corticospinal excitability under cathodal stimulation.
Initial studies showed that the excitability-modulating effects of anodal tDCS outlasts the stimulation for up to 90 min [14, 63]. In addition to the acute effects, meaning the ability to modify excitability of the neurons, tDCS displays after-effects such as long term potentiation (LTP)-like plasticity in the human motor cortex, which can last for more than 24 h after stimulation [71, 72]. Accordingly, a review on physiological mechanisms of tDCS concluded that the effects are multifactorial and associated with GABAergic, serotonergic, glutamatergic, dopaminergic and cholinergic activity modulation [73]. For instance, Nitsche et al. [74] and Liebetanz et al. [75] showed that the after-effects of anodal and cathodal tDCS are influenced by an enhanced efficacy of the N-methyl-D-aspartate (NMDA) receptor, a member of the ionotropic glutamate receptors. This is of interest as NMDA receptors were shown to be involved in neuroplastic changes [76]. Moreover, recently published studies could show that the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionicacid receptor (AMPAR), a crucial protein for enhancing synaptic transmission, is associated with tDCS induced plasticity in rodents [77, 78]. Taken together, these findings suggest that tDCS-related effects are not based on a single mechanism and involve a cascade of events at a molecular as well as on cellular level [73]. Generally, various parameters such as the current intensity, stimulation length and the number of sessions influence the duration of tDCS after-effects. However, the link between stimulation duration, current intensity and induced after-effects are more complex and increasing current strength [70, 79] or the stimulation duration [71, 80] do not necessarily show greater effectiveness. For instance, a study demonstrated that tDCS excitability effects are not linearly correlated with increasing current intensity [81].
Overall, conventional tDCS is known to be well-tolerated and with an applied current of 1–2 mA and a duration up to 20 min considered safe [82, 83]. Regarding HD-tDCS approaches, a study from Turski and colleagues [84] showed that 20 daily sessions of HD-tDCS in healthy adults administered over a variety of brain regions are safe and well tolerated. Concerning adverse effects (AE), a review concluded that most AEs are described as mild and short-lasting after stimulation [85].
3.1 Methods
3.1.1 Search strategy
This narrative review focused on the effects of tDCS aimed at the prefrontal cortex to influence food intake, food craving and body weight in healthy persons of different BMI groups. Only studies with tDCS as NIBS intervention were included in order to increase comparability of the already heterogeneous study designs. We sought to answer which tDCS protocols were able to influence eating behavior-related outcomes and show the most promising effects on weight-loss. In addition, this work elaborates possible modulators and limitations that influence tDCS effects with respect to eating behavior. To identify relevant studies, a two-staged literature search was carried out. First, an online search was conducted using the online databases Pubmed and Web of Science to cover articles published up to August 2021 with no starting date. For the PubMed search, the following search terms were used: "Transcranial Direct Current Stimulation"[Mesh] OR "transcranial direct current stimulation"[tw] OR "tDCS"[tw] OR "non invasive brain stimulation"[tw] OR "non-invasive brain stimulation"[tw] OR "NIBS"[tw] OR "brain stimulation"[tw] OR "neurostimulation"[tw] OR "neuromodulation"[tw] AND "Energy Intake"[Mesh] OR "Appetite"[Mesh] OR "eating behavior"[tw] OR "energy intake"[tw] OR "calorie consumption*"[tw] OR "caloric intake"[tw] OR "food addiction"[tw] OR "weight loss"[tw] OR "food craving*"[tw] OR "binge eating disorder"[tw] OR "food consumption"[tw] OR "appetite"[tw]. For the Web of Science search, the following search terms were used: (((((((TI = (transcranial direct current stimulation)) OR TI = (tDCS)) OR TI = (non invasive brain stimulation)) OR TI = (non-invasive brain stimulation)) OR TI = (neuromodulation)) OR TI = (neurostimulation)) OR TI = (brain stimulation)) OR TI = (NIBS) AND (((((((((TI = (eating behavior)) OR TI = (energy intake)) OR TI = (calorie consumption*)) OR TI = (caloric intake)) OR TI = (food addiction)) OR TI = (weight loss)) OR TI = (food craving)) OR TI = (binge-eating disorder)) OR TI = (food consumption)) OR TI = (appetite). Furthermore, review articles and meta-analysis were examined to identify additional articles. The identified studies were subsequently hand screened by reading the title and abstract and included in the study if they matched the research topic of our review. The remaining articles were evaluated in detail. A graphical depiction of the inclusion and exclusion process is illustrated in Fig. 2.
3.1.2 Selection criteria
In this review, all studies were included that met the following criteria: 1) randomized controlled trials and controlled clinical trials 2) tDCS was used as form of a non-invasive brain stimulation 3) food intake, hunger or appetite/desire to eat ratings, food craving changes or changes in body weight were measured 4) studies were placebo-controlled (i.e. sham stimulation as control condition). We excluded studies based on following criteria: 1) meta-analyses, reviews, case studies or meeting abstracts, 2) if participants were diagnosed with psychiatric disorders except for binge-eating disorder, 3) stimulation target other than the prefrontal cortex 4) animal studies, 5) studies not written in English.
3.1.3 Data extraction
Extracted data included the most important stimulation parameters: number of tDCS sessions, stimulation site (anode and cathode placement), current intensity, electrode size, duration of each session (min), total stimulation duration (min), tDCS montage (e.g. unilateral, bilateral), tDCS-Form (e.g. bipolar, HD-tDCS), between or within study-design and multi-session or single-session approach. Moreover, participant characteristics were included: population characteristics, number of subjects, and number of male and female participants, BMI and age. Lastly, factors related to the outcome measures were included (fasting time prior appointment) as well as the most important study results for the research question (weight loss, craving measurements, food intake measurements, desire to eat/hunger).
The literature search identified 25 studies that met the inclusion criteria. Most studies (n = 23) targeted the DLPFC (n = 7 targeted the left DLPFC, n = 15 targeted the right DLPFC, n = 1 targeted both, left and right DLPFC), while two studies targeted the right IFG.
4 tDCS effects on food craving and desire to eat
Food craving and food cue reactivity have shown to predict caloric intake and weight gain [87]. Persons with elevated BMI and BED display higher food craving [88,89,90]. Even though the theory that foods can trigger an addictive process remains controversial, neurobiological evidence shows similar neural pathways between food craving and drug craving [12, 91, 92]. These include regions implicated in homeostasis, motivation, reward and emotions, which are deeply located in the subcortical part of the brain and are therefore difficult to reach with conventional tDCS methods. Nonetheless, cortical regions, such as the frontal cortex, can be easily targeted with tDCS and are functionally connected to other regions in the brain [14, 63, 93, 94]. It is hypothesized that targeting frontal brain areas improves cognitive control and response inhibition [95,96,97]. Consistent with this, a recent meta-analysis confirmed that a single tDCS application has a significant overall effect on inhibitory control [98].
Food craving can be measured using questionnaires such as the Food Cravings Questionnaire–State (FCQ-S) or by using a visual analogue scale (VAS). Other craving measurements include visual food reactivity tasks where images display different kinds of food (e.g. savory foods, dessert and non-sweet carbohydrates) on a screen and participants are asked to rate on a VAS to what degree they “like” and “want” the presented food before and immediately after stimulation. For evaluation, the “wanting” scores are used, as these are considered to reflect food craving. Figure 3 provides an overview of the tools used to assess eating behavior in response to tDCS. The majority of tDCS studies examining the effects on food craving targeted the right DLPFC [99,100,101,102,103,104,105,106,107,108,109,110], as it is associated with inhibitory control and reward-based learning [10, 111]. Some trials aimed to enhance self-control by stimulating the left DLPFC [112,113,114, 116, 157], or targeting both sites [116] by using anodal stimulation at the right and left DLPFC, respectively.
One study, however, stimulated specifically the right IFG [114], which is involved in response inhibition [117]. Recent findings show that anodal tDCS over the IFG can facilitate response inhibition by modulating neural activity and functional connectivity to other brain regions [118]. However, anodal stimulation of the right IFG had no effect on food craving [114]. See Table 1 for an overview of the study population and design and Supplementary Table 1, which provides an overview of the targeted brain areas and stimulation parameters. Table 2 presents the individual study findings summarized in this review.
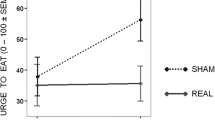
Overall, the main findings of tDCS on food craving show mixed effects, varying from significant reductions in food craving by active tDCS [99, 102, 104, 110, 116] to null findings [100, 101, 103, 105, 107,108,109, 113, 114, 116] or showing only significance for a specific group of individuals [106]. Even an opposite effect, favoring sham over anodal tDCS of the right DLPFC was shown in a single-session study [103]. However, these findings were mostly based on a subscale “craving as a physiological state” in the FCQ-S questionnaire, which contains statements about objectively determinable hunger, such as feeling weak as a result of food deprivation. When excluding this subscale from the analysis, the differences between sham and active tDCS were no longer significant. Moreover, the study used next to the FCQ-S a modified Food Challenge Task (FCT) to measure food craving which did not reveal differences between sham and active tDCS [103].
In a clinical trial from Burgess et al. [99], food craving and the desire to binge-eat was reduced after a single stimulation of anodal tDCS compared to sham targeting the right DLPFC. This effect was sex-specific, showing that men were more responsive compared to women regarding tDCS effects on desire to binge-eat and food craving [99]. Sex-effects were also reported by Ray et al. [106], where a reduction in food craving was only displayed in women with low attentional impulsivity. Other studies have also demonstrated sex-specific effects of tDCS on different cognitive domains. For instance, one study revealed that men benefit from tDCS stimulation of the left DLPFC, whereas women profit from right DLPFC stimulation in terms of verbal working memory [119]. Among the various explanations discussed for the differential effects of tDCS in men and women is the influence of sex hormones and neurotransmitters [120].
Despite the heterogeneous results of tDCS on food craving [100, 101, 103, 105, 107,108,109, 113, 114, 116], some studies identified an effect of active tDCS on food craving and appetite on specific foods [99, 102,103,104, 121]. In particular, the reduction in cravings for sweet foods such as desserts appear to be fairly consistent and has been observed in several tDCS studies aimed at the right DLPFC. Therby, it does not seem to matter whether the subjects are obese or not, as the effects were demonstrated in both subjects of normal-weight and obesity. Most studies showing this effect examined participants experiencing frequent cravings, BED, or sub-BED [99, 102,103,104]. Nevertheless, a study with healthy normal-weight men also showed decreased appetite for sweets after 8 consecutive anodal tDCS sessions [121]. Contrary, a study including overweight and obese subjects without eating disorder could not demonstrate this effect on sweet food after anodal tDCS [109]. For other food categories such as savory food, the data are not as consistent [102, 104]. However, cravings for chocolate and sweets have been shown to be more prevalent compared to craving for savory foods [122] which may explain the mixed results for sweet foods compared to other food categories.
Taken together, the current literature of tDCS studies targeting the left or right DLPFC show conflicting results with respect to food craving. One possible explanation for this variability could be the highly diverse study population. For instance, studies showing a diminished craving for sweet foods included mostly participants with either frequent food craving [102,103,104], BED or subBED [99], thus demonstrating a study population that exhibits dysregulated eating behaviors. A recently published meta-analysis evaluated the effects of modulators in tDCS studies on food and substance craving and concluded that the stimulation site (anodal left or right DLPFC) and current intensity (1 or 2 mA) do not influence tDCS outcomes [123]. However, stimulation duration made a significant difference, meaning that a longer total stimulation time was associated with a stronger craving reduction [123]. In addition, studies with multiple sessions showed a better effect on craving than stimulations with only one session. [123, 124].
5 tDCS modulating food intake
Much effort is being made to evaluate tDCS protocols on food intake measures in order to establish new treatment strategies for persons with obesity and eating disorders. So far, most studies evaluated anodal tDCS over the right DLPFC (for overview see Supplementary Table 1). For this purpose, studies use mostly snack tests [99, 101,102,103, 105, 106, 108,109,110, 112, 116, 125], vending machine paradigms [112, 126], ad libitum test buffets [121, 127], dietary records or dietary recalls [113, 128,129,130] (see Fig. 3, Table 2).
5.1 Snack test
For in-lab food consumption, snack tests are commonly used. Participants are usually left alone in a test room for a certain amount of time (~ between 10 and 20 min) to consume the served snacks ad libitum. The consumed calories are calculated afterwards. In some studies, the true reason for the snack test was masked. Subjects are then asked to rate the snack for taste and palatability [99, 106, 109, 112, 125], which is a valid measure of food consumption [131].
Active tDCS aimed at the right DLPFC reduced craving ratings and appetite for highly palatable food [99, 102,103,104, 121]. Actual food consumption (i.e. chocolate), on the other hand, was increased after active tDCS compared to sham in participants who reported to frequently crave chocolate [125]. However, in this study, tDCS was directed at the right IFG, whereas the aforementioned studies investigated food craving in response to tDCS covering the right DLPFC, making a direct comparison of these study results difficult.
A multi-session study showed after four weeks of anodal tDCS over the left DLPFC (15 sessions in total), that obese participants consumed less snacks compared to subjects of the sham group, particularly less sweet foods such as candy [112]. Interestingly, no significant lower consumption of snacks was observed after only three consecutive tDCS sessions between the sham and active tDCS group [112]. This may indicate that the effects of tDCS become apparent only after a certain number of sessions. In fact, a recent meta-analysis confirmed that repeated tDCS and rTMS sessions have a stronger effect on food consumption than single-session studies [124]. Nevertheless, studies using single-session tDCS protocols seem to be effective as well [99, 110, 116]. However, a large study including 172 participants in the analysis failed to show a reduced snack food intake after a single application of anodal tDCS targeting the right DLPFC [108]. Other studies also failed to demonstrate reduced snack intake after anodal tDCS in single-session approaches [101, 102, 106]. Furthermore, the expectation of receiving active stimulation seemed to influence the actual food intake. Informing participants that they will receive active tDCS resulted in decreased food intake, regardless of whether subjects actually received real or sham tDCS [105].
5.2 Vending machine paradigm
Vending machine paradigms can be used to accurately measure food intake throughout the day [132, 133]. In this approach, each participant has ad libitum access to a vending machine for a specified period of time (e.g. 23.5 h after completion of the tDCS session). The vending machine is filled with a variety of foods previously selected based on personal ratings in a Food Preference Questionnaire [134], as well as additional beverages. For food intake, subjects are asked to consume all meals in a specific room and are not allowed to use electronic devices. Moreover, participants have to return any food not consumed in order to evaluate total caloric intake.
Two studies by the same working group used a vending machine paradigm to assess complete caloric intake during three days of consecutive tDCS in obese participants [112, 126]. Thereby, subjects were in an inpatient setting for nine [126] and 11 days [112] and received a weight-maintaining diet for the first five [126] and seven [112] days. Subsequently, active anodal tDCS and cathodal tDCS, respectively, or sham stimulations targeting the left DLPFC were performed in the morning on three consecutive days. After the respective tDCS sessions, participants had the opportunity to eat ad libitum products of a vending machine. However, no effect was observed on caloric intake between active and sham stimulation [112, 126]. Gluck et al. [126] though, identified a trend towards lower total caloric intake in response to anodal compared to cathodal stimulation. In addition, the anodal tDCS group consumed significantly fewer calories from fat and soda compared to participants receiving cathodal tDCS [126]. When interpreting these data, the inpatient setting should taken into account. Robinson et al. [135] showed that increased awareness of being observed caused reductions in caloric consumption.
5.3 Test buffet
In contrast to snack tests, a test buffet usually contains a larger selection of foods. Here, too, the participants have the opportunity to consume the foods presented ad libitum for a certain period of time, which may be longer as during a snack test.
To our knowledge, two studies assessed food intake in response to tDCS using a test buffet. Jauch-Chara et al. [121] showed that eight days of consecutive anodal tDCS targeted at the right DLPFC could reduce caloric intake in young, normal-weight men compared to eight days of sham tDCS in a crossover-design study. The reduced caloric intake was mostly due to a reduced intake of carbohydrates [121]. In another study including women with obesity, subjects received in a random order single anodal, cathodal, and sham tDCS aimed at the left DLPFC with subsequent ad libitum ad libitum buffet after stimulation. Results did not show differences in the overall caloric intake between conditions [127].
5.4 Dietary record and dietary recall
Some intervention studies use self-reported dietary records to assess participants' dietary intake in their free-living environment. In this process, subjects typically weigh all the foods and beverages they consumed over a specified period of time. A dietary recall, on the other hand, usually consists of a guided interview by which participants list all foods and beverages consumed in the last 24 h. Several studies examined the effects of tDCS on food intake behaviors using these assessments.
For instance, one study aimed at enhancing the right DLPFC in morbid obese patients using a HD multichannel tDCS configuration to increase focality. Therby, patients received four days of consecutive tDCS combined with a cognitive training prior to bariatric surgery. Active stimulated patients reduced their caloric intake measured by a food diary compared to subjects who received sham. Interestingly, this effect was stronger at follow-up [128]. Another multi-session tDCS study administered daily anodal or sham tDCS targeting the right DLPFC over four weeks (20 sessions in total) in overweight and obese subjects. Next to tDCS as an intervention, participants had to follow a hypocaloric diet. No differences between stimulation conditions were observed on food intake based on a 3-day weighed dietary record [129]. Other studies failed to show an effect of anodal tDCS on food intake as well [113].
Together, these findings show that the effects of tDCS on caloric intake are highly variable. A meta-analytic review concluded that for single-session tDCS and rTMS approaches targeting the DLPFC no causal effect on food consumption could be confirmed [136]. In addition, a more recent meta-analysis from Song et al. [124] evaluated the effects of excitatory tDCS and rTMS aimed at the DLPFC on craving and consumption in persons suffering from eating disorder, obesity or drug addiction. There, a significant effect of non-invasive neurostimulation on consumption, including drug consumption such as alcohol and nicotine, was found for single-session studies as well as for multi-session approaches. Restricting the analysis to food intake revealed a significant reduction in consumption with a medium effect size [124].
6 The impact of tDCS on body weight
The ultimate goal of evaluating novel tDCS paradigms is to achieve clinical relevant effects as successful weight loss or body weight maintenance. A number of studies assessed the impact of tDCS on body weight changes [112,113,114, 116, 121, 126, 129, 157]. Here, only few trials stimulated the right DLPFC [121, 129] while most studies conducted tDCS of the left DLPFC [112,113,114, 116, 126, 157].
In an inpatient-design study, three days of consecutive tDCS of the left DLPFC did not influence body weight in the active tDCS groups (anodal and cathodal) compared to subjects receiving sham stimulation [126]. Despite the lack of differences between active and sham tDCS, subgroup-analysis comparing anodal vs. cathodal stimulation revealed that obese subjects receiving anodal tDCS showed a significant higher weight loss [126]. One study published at a later time by the same research group evaluated only the effects of anodal vs. sham tDCS aimed at the left DLPFC and showed no differences in weight change after 15 sessions of stimulation in the anodal tDCS group compared to the sham group [112]. Consistent with these results, eight days of consecutive active anodal tDCS aimed at the right DLPFC had no impact on body weight compared to sham stimulation in a crossover-design study. However, the study was conducted in healthy normal-weight men [121]. Hence, no conclusion can be drawn whether persons with obesity could reduce their body weight with this tDCS protocol.
In addition to tDCS as a stand-alone intervention, there are also studies examining the effects of tDCS in combination with a hypocaloric diet on weight loss. A recent trial conducted from Amo Usanos et al. [115] involved overweight and obese women and investigated the effects of a 4-week trial with a total of eight tDCS sessions. Here, participants received in the first week five consecutive tDCS sessions and in the second week three tDCS sessions combined with a hypocaloric diet followed by two weeks of a hypocaloric diet only. Both groups reduced body weight but the active group showed a significant greater reduction than the sham group throughout the study [116]. Another recent trial examining tDCS effects in combination with a hypocaloric diet in overweight and obese participants could not confirm these findings after 20 sessions of active or sham tDCS. Although the active group lost more weight, the differences did not reach statistical significance [129]. However, it should be mentioned that the two studies are difficult to compare, as different sides of the DLPFC were anodal stimulated (right [129] vs. left [116]). Moreover, in the study by Amo Usanos et al. [115] all subjects were exclusively female, whereas in the study by de Araujo et al. [129] 50% of the subjects were male, thus increasing interindividual variability.
We are not aware of any meta-analysis that examined the effects of tDCS on weight loss. Nevertheless, existing literature suggest that there are several modulators that contribute to the results of each study. Future studies should focus on the modulators affecting the outcomes of tDCS studies regarding weight loss.
7 Limitations
There are multiple factors and modulators which may account for the variability of the outcomes in studies investigating the effects of tDCS on eating behavior and weight-loss.
7.1 Single vs. multi-session approaches
First, some studies investigated tDCS effects using a single-session tDCS design while other trials used multi-session approaches (see Table 1 for overview). Meta-analytic approaches already elucidated that multi-session design studies have stronger effects compared to single-session stimulations regarding the reduction of food craving and consumption [123, 124].
7.2 Current strength
While most studies used 2 mA, some trials examinated tDCS effects on eating behavior using lower intensities [101, 107, 114, 121]. These studies did not report an effect of single session active tDCS vs. sham stimulation on food craving [101, 107, 114], food consumption [101, 121], desire to eat [101, 107, 114] or hunger [114, 121]. However, Jauch-Chara et al. [121] were able to detect significant effects on food intake and appetite scores after the completion of eight tDCS sessions using a DC of 1 mA. One possible explanation for null-findings after single-session tDCS could be an insufficient current strength. Before the current reaches the targeted brain region, the electric field distribution during tDCS is determined by several factors like the gyral depth, the thickness of the skull, and the cerebrospinal fluid as well as the distance from anode to cathode. This could be shown by using anatomically realistic finite element models (FEM) [137]. Moreover, other factors such as head fat were shown to affect tDCS electric current density across the brain [138]. In addition, a recently published study reported that only about 25% of the applied current reaches the brain [139] and Hall and Lowe [140] concluded that 1 mA is an insufficient current for affecting brain networks relevantly [139]. However, it is crucial to mention that this conclusion was drawn based on human post mortem brain tissue. Contrary to the prior conclusion, a study investigating the effects of different stimulation intensities (0.5 – 2.0 mA) for anodal and cathodal tDCS found that lower intensities (0.5 and 1 mA) displayed equal effects in the excitability of the motor cortex. Moreover the researchers showed that stimulation effects were not correlated with increased DC intensities [81].
7.3 tDCS method and further directions
Non-invasive brain stimulation techniques such as tDCS can alter cortical activity through electrical current with the potential to induce long-lasting behavioral effects. A majority of the tDCS studies used conventional tDCS with two large sponge electrodes. However, this stimulation montage results in a diffuse brain current flow [66]. Novel technologies such as HD-tDCS overcome the diffuse electric -field by using multiple smaller electrodes, which have shown to increase focality [66, 68, 141] (see Fig. 1). First results are promising for multisession approaches [128].
In terms of eating behavior regulation and inhibitory control, the DLPFC represents an important target area for stimulation, and indeed applying tDCS to this brain region has been shown to reduce food craving and intake [124]. Nevertheless, it is well-known that the human brain is organized in functional networks rather than working in isolation [142,143,144,145] with the DLPFC being only one player in the regulation of human eating behavior [146]. Therefore, brain stimulation methods are needed that allow modulation of network activity as a whole rather than stimulating isolated brain regions (see [94] for review). Fischer et al. [147] could show that multifocal tDCS targeting the left M1 and its associated network more than doubled the increase of the excitability over time in the aimed brain area compared to traditional tDCS. Moreover, Dagan et al. [148] compared the effects of tDCS over M1 (single-target) to a multi-target stimulation of M1 and the DLPFC using HD-tDCS on cognitive and motor function in patients with Parkinson’s disease. Here, a single-session of multi-target stimulation of both brain areas showed a significant improvement of the outcomes compared to the single-target intervention. Overall, network-targeted tDCS is a promising method to enhance tDCS effects. Further investigations are needed to determine if these effects can be demonstrated for other brain networks and at the behavioral level.
7.4 tDCS combined with different interventions
Another explanation of the heterogeneity of tDCS outcomes include methodological variability. Thereby it has been shown that the administration of a task, for instance go/nogo tasks, may play a role in the different outcomes between tDCS studies [149]. Moreover, some studies combine tDCS together with a hypocaloric diet or exercise. A recent review concluded that a combination of tDCS and aerobic exercise may have beneficial synergistic effects on cognition [150]. However, it is not clear if this is also the case for eating behavior related tDCS outcomes.
7.5 Stimulation site of tDCS
Moreover, one might hypothesize that the different tDCS effects on food consumption and craving are based on lateralization effects, as different sides of the DLPFC (left vs. right) were stimulated. Recent meta-analyses examined lateralization as a possible modulator of tDCS outcomes. A meta-analysis evaluating rTMS and tDCS effects on food consumption and food craving showed that the effect size was significantly greater for studies targeting the left DLPFC [151]. In contrast, Song et al. [124] who examined the effects of rTMS and tDCS on cravings and consumption of food and substances did not identify this lateralization effect, even when the analysis was limited to food cravings only. This is in line with the meta-analysis by Chen et al. [123] examining the effects of tDCS on food and substance craving such as nicotine, alcohol and drugs, which revealed no significant difference between the right and the left DLPFC but indicated a greater effect size for the right DLPFC. Taken together, these findings could point to a more prominent role of the right DLPFC on food craving.
7.6 Placebo-effect
In addition, it is important to keep in mind that results differ when subjects believe or perceive that they received active tDCS stimulation. In this context, Goldman et al. [102] showed that single anodal stimulation aimed at the right DLPFC resulted in reduced food craving ratings. However, subjects were able to guess the applied stimulation condition (real or sham tDCS) in 79% of the cases, indicating that blinding was unsuccessful and participants could identify the stimulation they had received [102]. Accordingly, Ray et al. [105] controlled for treatment expectation in a tDCS study aimed at the right DLPFC. The effects on food craving and consumption were investigated after participants were either told they receive active anodal tDCS or placebo stimulation. In reality, 50% of the subjects received in a randomized order sham and 50% received true stimulation. Interestingly, subjects told that they were stimulated with active tDCS craved less compared to participants who expected to receive sham stimulation. Hence, it did not matter if they actually received sham or active stimulation. There was no significant difference in food craving between tDCS conditions, demonstrating the power of expectation and the need to proper control tDCS experiments [105].
7.7 Inter-individual variability influencing tDCS outcomes
In addition, the high interindividual variability makes it difficult to draw conclusions about the modulation of food craving and food intake by tDCS. One reason for the inconsistency may be the genetic predisposition of the subjects. Catechol-O-methyl transferase (COMT) enzymes are crucial in the degradation of dopamine in the PFC [152]. A single nucleotide polymorphism (SNP) Valine158Methionine (Val158Met) in the COMT gene influences the enzyme’s activity and is linked with an altered function of the PFC. The enzyme’s activity is higher with the Val allele compared to the Met allele, resulting in a lower prefrontal dopamine signaling [152]. A tDCS study investigated COMT gene variability. Subjects received either 16 sessions (4 weeks) of anodal tDCS or sham stimulation, which was partly combined with hypocaloric diet. There was no difference in weight loss between the two groups at the end of the stimulation period. Actually, 77% of the subjects in the active tDCS group had regained weight at follow-up. In comparison, only 17% of the participants in the sham group had regained weight. Genetic analysis of COMT gene variability, however, showed that it was mainly the Met noncarriers that were responsible for this weight gain in the active group [113]. Other studies using tDCS already showed as well the interaction between tDCS and genetical determined variations [153, 154]. Besides the genetic predisposition which was shown to affect tDCS outcomes, sex also affects the modulatory effects of tDCS, as already discussed above [106, 119, 120]. Moreover, other factors such as the baseline state of the activated brain area can determine the effects of brain stimulation ([155]; see [156] and [157] for review). Thus, the effects of neurostimulation are already at a physiological state subject to individual differences.
8 Conclusion
Over the past decade, there is an increasing interest to use tDCS as a novel treatment approach for obesity and eating disorders. It is evident that multisession studies are more effective to reduce food craving and consumption than single-session approaches [123, 124]. Moreover, results from various studies suggest that tDCS has a positive impact on food craving, particularly for specific foods such as sweets. Most trials were conducted with very limited sample size, which makes it difficult to draw firm conclusions. Yet overall, the literature on tDCS effects on food intake and craving display a mix of positive and null-findings. In addition, the exact mechanisms behind tDCS effects remain unclear. Further research should focus on a combination of neuroimaging techniques such as fMRI and tDCS in order to provide underlying mechanisms of anodal and cathodal stimulation. The fast growing literature in brain research elucidated that brain regions do not operate in isolation but interact constantly with each other [142,143,144,145]. Multifocal tDCS targeting a whole network increases excitability in the targeted brain area more than twofold over time compared to conventional tDCS [147]. Therefore, multifocal tDCS arrangements with smaller electrodes could facilitate to stimulate whole brain networks and thus not only target the DLPFC but indirectly stimulate other brain structures involved in eating behavior regulation.
Abbreviations
- AeCi:
-
Anodal-excitation and cathodal-inhibition
- AF:
-
Adverse effects
- AMPAR:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionicacid receptor
- BED:
-
Binge eating disorder
- BMI:
-
Body-Mass-Index
- COMT:
-
Catechol-O-methyl transferase
- DC:
-
Direct current
- DLPFC:
-
Dorsolateral prefrontal cortex
- FCQ-S:
-
Food Craving Questionnaire – State
- fMRI:
-
Functional MRI
- HD:
-
High-definition
- IFG:
-
Inferior frontal gyrus
- IR:
-
Insulin resistance
- LTP:
-
Long term potentiation
- M1:
-
Primary motor cortex
- NIBS:
-
Non-invasive brain stimulation
- NMDA:
-
N-methyl-D-aspartate
- PFC:
-
Prefrontal cortex
- rTMS:
-
Repetitive transcranial magnetic stimulation
- SNP:
-
Single nucleotide polymorphism
- T2D:
-
Type 2 diabetes
- tDCS:
-
Transcranial direct current stimulation
- Val158Met:
-
Valine158Methionine
- VAS:
-
Visual Analogue Scale
References
Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14.
Pozza C, Isidori AM. What’s Behind the Obesity Epidemic. In: Imaging in Bariatric Surgery. Springer, Cham; 2018: 1–8.
Mitchell NS, Catenacci VA, Wyatt HR, et al. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34:717–32.
World Health Organization. Obesity and overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (1 December 2021, date last accessed).
Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab. 2016;23:48–62.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Ma Y, Ajnakina O, Steptoe A, et al. Higher risk of dementia in English older individuals who are overweight or obese. Int J Epidemiol. 2020.
Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013;7:14–24.
Aronne LJ, Nelinson DS, Lillo JL. Obesity as a disease state: A new paradigm for diagnosis and treatment. Clin Cornerstone. 2009;9:9–29.
Lowe CJ, Reichelt AC, Hall PA. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn Sci (Regul Ed ). 2019;23:349–61.
Berthoud HR, Münzberg H, Morrison CD. Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology. 2017;152:1728–38.
Tang DW, Fellows LK, Small DM, et al. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–24.
Donofry SD, Stillman CM, Erickson KI. A review of the relationship between eating behavior, obesity and functional brain network organization. Soc Cogn Affect Neurosci. 2020;15:1157–81.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol (Lond ). 2000;527(Pt 3):633–9.
Bikson M, Grossman P, Thomas C, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016;9:641–61.
Mameli F, Fumagalli M, Ferrucci R, et al. Transcranial Direct Current Stimulation and Cognition in the Elderly. In: The Stimulated Brain. Elsevier, 371–95.
Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–23.
Zhao H, Qiao L, Fan D, et al. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (tDCS): Clinical Applications and Safety Concerns. Front Psychol. 2017;8:685.
Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–96.
Chen R, Seitz RJ. Changing cortical excitability with low-frequency magnetic stimulation. Neurology. 2001;57:379–80.
Filmer HL, Mattingley JB, Dux PE. Modulating brain activity and behaviour with tDCS: Rumours of its death have been greatly exaggerated. Cortex. 2020;123:141–51.
Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci (Regul Ed ). 2003;7:415–23.
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69.
Ruocco AC, Rodrigo AH, Lam J, et al. A problem-solving task specialized for functional neuroimaging: validation of the Scarborough adaptation of the Tower of London (S-TOL) using near-infrared spectroscopy. Front Hum Neurosci. 2014;8:185.
Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–8.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Braver TS, Barch DM, Gray JR, et al. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–36.
Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62.
Sleezer BJ, LoConte GA, Castagno MD, et al. Neuronal responses support a role for orbitofrontal cortex in cognitive set reconfiguration. Eur J Neurosci. 2017;45:940–51.
Ridderinkhof KR, Ullsperger M, Crone EA, et al. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7.
Tops M, Boksem MAS. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330.
Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–87.
Hollmann M, Hellrung L, Pleger B, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond). 2012;36:648–55.
Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–6.
Siep N, Roefs A, Roebroeck A, et al. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60:213–20.
Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–703.
Le DSNT, Pannacciulli N, Chen K, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84:725–31.
Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–46.
Wever MCM, van Meer F, Charbonnier L, et al. Associations between ghrelin and leptin and neural food cue reactivity in a fasted and sated state. Neuroimage. 2021;240:118374.
Charbonnier L, van Meer F, Johnstone AM, et al. Effects of hunger state on the brain responses to food cues across the life span. Neuroimage. 2018;171:246–55.
Roberts CA, Giesbrecht T, Fallon N, et al. A Systematic Review and Activation Likelihood Estimation Meta-Analysis of fMRI Studies on Sweet Taste in Humans. J Nutr. 2020;150:1619–30.
Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127–128:64–90.
Heni M, Kullmann S, Ketterer C, et al. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp. 2014;35:918–28.
Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8:e60393.
Le DSN, Pannacciulli N, Chen K, et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–9.
Veit R, Schag K, Schopf E, et al. Diminished prefrontal cortex activation in patients with binge eating disorder associates with trait impulsivity and improves after impulsivity-focused treatment based on a randomized controlled IMPULS trial. Neuroimage Clin. 2021;30:102679.
Anzman SL, Birch LL. Low inhibitory control and restrictive feeding practices predict weight outcomes. J Pediatr. 2009;155:651–6.
Nederkoorn C, Houben K, Hofmann W, et al. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010;29:389–93.
Allan JL, Johnston M, Campbell N. Unintentional eating. What determines goal-incongruent chocolate consumption? Appetite. 2010;54:422–25.
Cornier M-A, Salzberg AK, Endly DC, et al. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–43.
Veit R, Horstman LI, Hege MA, et al. Health, pleasure, and fullness: changing mindset affects brain responses and portion size selection in adults with overweight and obesity. Int J Obes (Lond). 2020;44:428–37.
DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond). 2007;31:440–8.
Weygandt M, Mai K, Dommes E, et al. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–78.
Weygandt M, Mai K, Dommes E, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–27.
Goldman RL, Canterberry M, Borckardt JJ, et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring). 2013;21:2189–96.
Kohl SH, Veit R, Spetter MS, et al. Real-time fMRI neurofeedback training to improve eating behavior by self-regulation of the dorsolateral prefrontal cortex: A randomized controlled trial in overweight and obese subjects. Neuroimage. 2019;191:596–609.
Spetter MS, Malekshahi R, Birbaumer N, et al. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real-time fMRI feedback study. Appetite. 2017;112:188–95.
Lefaucheur JP, Antal A, Ahdab R, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1:337–44.
Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–5.
McClelland J, Bozhilova N, Campbell I, et al. A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. Eur Eat Disord Rev. 2013;21:436–55.
Fregni F, Marcondes R, Boggio PS, et al. Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol. 2006;13:996–1001.
Moreno-Duarte I, Gebodh N, Schestatsky P, et al. Transcranial Electrical Stimulation. In: The Stimulated Brain. Elsevier; 2014: 35–59.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901.
Rostami M, Golesorkhi M, Ekhtiari H. Methodological dimensions of transcranial brain stimulation with the electrical current in human. Basic Clin Neurosci. 2013;4:190–208.
Caparelli-Daquer EM, Zimmermann TJ, Mooshagian E, et al. A pilot study on effects of 4×1 high-definition tDCS on motor cortex excitability. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:735–8.
Datta A, Bansal V, Diaz J, et al. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(201–7):207.e1.
Dmochowski JP, Datta A, Bikson M, et al. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:46011.
Edwards D, Cortes M, Datta A, et al. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75.
Purpura DP, Mcmurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–85.
Batsikadze G, Moliadze V, Paulus W, et al. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol (Lond ). 2013;591:1987–2000.
Monte-Silva K, Kuo M-F, Hessenthaler S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–32.
Bikson M, Paulus W, Esmaeilpour Z, et al. Mechanisms of Acute and After Effects of Transcranial Direct Current Stimulation. In: Knotkova H, Nitsche MA, Bikson M et al. (eds.). Practical Guide to Transcranial Direct Current Stimulation: Principles, Procedures and Applications. Cham: Springer International Publishing; 2019: 81–113.
Medeiros LF, de Souza ICC, Vidor LP, et al. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 2012;3:110.
Nitsche MA, Fricke K, Henschke U, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol (Lond ). 2003;553:293–301.
Liebetanz D, Nitsche MA, Tergau F, et al. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain: J Neur. 2002;125:2238–47.
Bennett M. The concept of long term potentiation of transmission at synapses. Prog Neurobiol. 2000;60:109–37.
Martins CW, Melo Rodrigues LC, de, Nitsche MA, et al. AMPA receptors are involved in prefrontal direct current stimulation effects on long-term working memory and GAP-43 expression. Behav Brain Res. 2019;362:208–12.
Stafford J, Brownlow ML, Qualley A, et al. AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiol Learn Mem. 2018;150:36–41.
Kidgell DJ, Daly RM, Young K, et al. Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast. 2013;2013:603502.
Monte-Silva K, Kuo MF, Liebetanz D, et al. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol. 2010;103:1735–40.
Jamil A, Batsikadze G, Kuo HI, et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol. 2017;595:1273–88.
Reinhart RMG, Cosman JD, Fukuda K, et al. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys. 2017;79:3–23.
Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53.
Turski CA, Kessler-Jones A, Chow C, et al. Extended Multiple-Field High-Definition transcranial direct current stimulation (HD-tDCS) is well tolerated and safe in healthy adults. Restor Neurol Neurosci. 2017;35:631–42.
Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: A review. Clin Neurophysiol Pract. 2017;2:19–25.
Shekhawat GS, Sundram F, Bikson M, et al. Intensity, Duration, and Location of High-Definition Transcranial Direct Current Stimulation for Tinnitus Relief. Neurorehabil Neural Repair. 2016;30:349–59.
Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev. 2016;17:159–77.
Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J Consult Clin Psychol. 2000;68:95–102.
Waters A, Hill A, Waller G. Bulimics’ responses to food cravings: is binge-eating a product of hunger or emotional state? Behav Res Ther. 2001;39:877–86.
White MA, Grilo CM. Psychometric properties of the Food Craving Inventory among obese patients with binge eating disorder. Eat Behav. 2005;6:239–45.
Constant A, Moirand R, Thibault R, et al. Meeting of Minds around Food Addiction: Insights from Addiction Medicine, Nutrition, Psychology, and Neurosciences. Nutrients. 2020;12.
Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–79.
Nitsche MA, Liebetanz D, Lang N, et al. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2.
Ruffini G, Wendling F, Sanchez-Todo R, et al. Targeting brain networks with multichannel transcranial current stimulation (tCS). Curr Opin Biomed Eng. 2018;8:70–7.
Wiegand A, Sommer A, Nieratschker V, et al. Improvement of cognitive control and stabilization of affect by prefrontal transcranial direct current stimulation (tDCS). Sci Rep. 2019;9:6797.
Jacobson L, Javitt DC, Lavidor M. Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci. 2011;23:3380–7.
Stramaccia DF, Penolazzi B, Sartori G, et al. Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Exp Brain Res. 2015;233:2283–90.
Schroeder PA, Schwippel T, Wolz I, et al. Meta-analysis of the effects of transcranial direct current stimulation on inhibitory control. Brain Stimul. 2020;13:1159–67.
Burgess EE, Sylvester MD, Morse KE, et al. Effects of transcranial direct current stimulation (tDCS) on binge eating disorder. Int J Eat Disord. 2016;49:930–6.
Beaumont JD, Davis D, Dalton M, et al. The effect of transcranial direct current stimulation (tDCS) on food craving, reward and appetite in a healthy population. Appetite. 2020;157:105004.
Georgii C, Goldhofer P, Meule A, et al. Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiol Behav. 2017;177:20–6.
Goldman RL, Borckardt JJ, Frohman HA, et al. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite. 2011;56:741–6.
Kekic M, McClelland J, Campbell I, et al. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite. 2014;78:55–62.
Ljubisavljevic M, Maxood K, Bjekic J, et al. Long-Term Effects of Repeated Prefrontal Cortex Transcranial Direct Current Stimulation (tDCS) on Food Craving in Normal and Overweight Young Adults. Brain Stimul. 2016;9:826–33.
Ray MK, Sylvester MD, Helton A, et al. The effect of expectation on transcranial direct current stimulation (tDCS) to suppress food craving and eating in individuals with overweight and obesity. Appetite. 2019;136:1–7.
Ray MK, Sylvester MD, Osborn L, et al. The critical role of cognitive-based trait differences in transcranial direct current stimulation (tDCS) suppression of food craving and eating in frank obesity. Appetite. 2017;116:568–74.
Sedgmond J, Chambers CD, Lawrence NS, et al. No evidence that prefrontal HD-tDCS influences cue-induced food craving. Behav Neurosci. 2020;134:369–83.
Sedgmond J, Lawrence NS, Verbruggen F, et al. Prefrontal brain stimulation during food-related inhibition training: effects on food craving, food consumption and inhibitory control. R Soc Open Sci. 2019;6:181186.
Stevens CE, Lausen MA, Wagstaff LE, et al. Effect of transcranial direct current stimulation (tDCS) on food craving and eating when using a control method that minimizes guessing of the real vs. control condition. Eat Weight Disord. 2020.
Lapenta OM, Di Sierve K, de Macedo EC, et al. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite. 2014;83:42–8.
Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–22.
Heinitz S, Reinhardt M, Piaggi P, et al. Neuromodulation directed at the prefrontal cortex of subjects with obesity reduces snack food intake and hunger in a randomized trial. Am J Clin Nutr. 2017;106:1347–57.
Fassini PG, Das SK, Magerowski G, et al. Noninvasive neuromodulation of the prefrontal cortex in young women with obesity: a randomized clinical trial. Int J Obes (Lond). 2020;44:1279–90.
Chen S, Jackson T, Dong D, et al. Exploring effects of single-session anodal tDCS over the inferior frontal gyrus on responses to food cues and food cravings among highly disinhibited restrained eaters: A preliminary study. Neurosci Lett. 2019;706:211–6.
Amo Usanos C, Valenzuela PL, de la Villa P, et al. Neuromodulation of the prefrontal cortex facilitates dietinduced weight loss in midlife women: a randomized, proof-of-concept clinical trial. Int J Obes. 2020;44:568–578. https://doi.org/10.1038/s41366-019-0486-x.
Fregni F, Orsati F, Pedrosa W, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51:34–41.
Lopez RB, Hofmann W, Wagner DD, et al. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014;25:1337–44.
Sandrini M, Xu B, Volochayev R, et al. Transcranial direct current stimulation facilitates response inhibition through dynamic modulation of the fronto-basal ganglia network. Brain Stimul. 2020;13:96–104.
Meiron O, Lavidor M. Unilateral prefrontal direct current stimulation effects are modulated by working memory load and gender. Brain Stimul. 2013;6:440–7.
Chaieb L, Antal A, Paulus W. Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Vis Neurosci. 2008;25:77–81.
Jauch-Chara K, Kistenmacher A, Herzog N, et al. Repetitive electric brain stimulation reduces food intake in humans. Am J Clin Nutr. 2014;100:1003–9.
Hill AJ. The psychology of food craving. Proc Nutr Soc. 2007;66:277–85.
Chen J, Qin J, He Q, et al. A Meta-Analysis of Transcranial Direct Current Stimulation on Substance and Food Craving: What Effect Do Modulators Have? Front Psychiatry. 2020;11:598.
Song S, Zilverstand A, Gui W, et al. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: A meta-analysis. Brain Stimul. 2019;12:606–18.
To C, Falcone M, Loughead J, et al. Got chocolate? Bilateral prefrontal cortex stimulation augments chocolate consumption. Appetite. 2018;131:28–35.
Gluck ME, Alonso-Alonso M, Piaggi P, et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring). 2015;23:2149–56.
Grundeis F, Brand C, Kumar S, et al. Non-invasive Prefrontal/Frontal Brain Stimulation Is Not Effective in Modulating Food Reappraisal Abilities or Calorie Consumption in Obese Females. Front Neurosci. 2017;11:334.
Forcano L, Castellano M, Cuenca-Royo A, et al. Prefrontal Cortex Neuromodulation Enhances Frontal Asymmetry and Reduces Caloric Intake in Patients with Morbid Obesity. Obesity (Silver Spring). 2020;28:696–705.
de Araujo C, Fitz RC, Natividade GR, et al. The effect of transcranial direct current stimulation along with a hypocaloric diet on weight loss in excessive weight people: A pilot randomized clinical trial. Clin Nutr ESPEN. 2020;40:68–76.
Montenegro RA, Okano AH, Cunha FA, et al. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite. 2012;58:333–8.
Robinson E, Haynes A, Hardman CA, et al. The bogus taste test: Validity as a measure of laboratory food intake. Appetite. 2017;116:223–31.
Venti CA, Votruba SB, Franks PW, et al. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91:343–8.
Rising R, Alger S, Boyce V, et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr. 1992;55:343–9.
Geiselman PJ, Anderson AM, Dowdy ML, et al. Reliability and Validity of a Macronutrient Self-Selection Paradigm and a Food Preference Questionnaire 11P. J. G. and M. L. D. are also affiliated with the Department of Psychology, Louisiana State University, Baton Rouge, LA. Physiol Behav. 1998;63:919–28.
Robinson E, Proctor M, Oldham M, et al. The effect of heightened awareness of observation on consumption of a multi-item laboratory test meal in females. Physiol Behav. 2016;163:129–35.
Lowe CJ, Vincent C, Hall PA. Effects of Noninvasive Brain Stimulation on Food Cravings and Consumption: A Meta-Analytic Review. Psychosom Med. 2017;79:2–13.
Opitz A, Paulus W, Will S, et al. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015;109:140–50.
Truong DQ, Magerowski G, Blackburn GL, et al. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: Impact of head fat and dose guidelines. Neuroimage Clin. 2013;2:759–66.
Vöröslakos M, Takeuchi Y, Brinyiczki K, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. 2018;9.
Hall PA, Lowe CJ. Cravings, currents and cadavers: What is the magnitude of tDCS effects on food craving outcomes? Nutr Neurosci. 2020;23:490–3.
Datta A, Elwassif M, Battaglia F, et al. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 2008;5:163–74.
Siegel M, Buschman TJ, Miller EK. Cortical information flow during flexible sensorimotor decisions. Science. 2015;348:1352–5.
Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. The Lancet Neurology. 2014;13:206–16.
Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci (Regul Ed). 2010;14:277–90.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Farr OM, Li C-SR, Mantzoros CS. Central nervous system regulation of eating: Insights from human brain imaging. Metabolism. 2016;65:699–713.
Fischer DB, Fried PJ, Ruffini G, et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage. 2017;157:34–44.
Dagan M, Herman T, Harrison R, et al. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord. 2018;33:642–6.
Dedoncker J, Brunoni AR, Baeken C, et al. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016;9:501–17.
Steinberg F, Pixa NH, Fregni F. A Review of Acute Aerobic Exercise and Transcranial Direct Current Stimulation Effects on Cognitive Functions and Their Potential Synergies. Front Hum Neurosci. 2018;12:534.
Hall PA, Lowe C, Vincent C. Brain stimulation effects on food cravings and consumption: an update on Lowe et al. (2017) and a Response to Generoso et al. (2017). Psychosom Med. 2017;79:839–42.
Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21.
Wiegand A, Nieratschker V, Plewnia C. Genetic Modulation of Transcranial Direct Current Stimulation Effects on Cognition. Front Hum Neurosci. 2016;10:651.
Plewnia C, Zwissler B, Längst I, et al. Effects of transcranial direct current stimulation (tDCS) on executive functions: influence of COMT Val/Met polymorphism. Cortex. 2013;49:1801–7.
Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci (Regul Ed). 2008;12:447–54.
Dayan E, Censor N, Buch ER, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16:838–44.
Krause B, Cohen KR. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci. 2014;8:25.
Marron EM, Viejo-Sobera R, Cuatrecasas G, et al. Prefronto-cerebellar neuromodulation affects appetite in obesity. Int J Obes (Lond). 2019;43:2119–24.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported in parts by a grant (01GI0925) from the Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ester, T., Kullmann, S. Neurobiological regulation of eating behavior: Evidence based on non-invasive brain stimulation. Rev Endocr Metab Disord 23, 753–772 (2022). https://doi.org/10.1007/s11154-021-09697-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-021-09697-3