Abstract
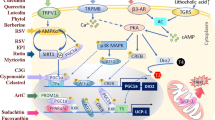
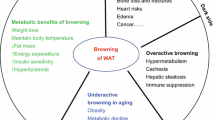
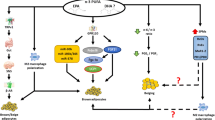
Obesity is a public health problem present in both developed and developing countries. The white adipose tissue (WAT) is the main deposit of lipids when there is an excess of energy. Its pathological growth is directly linked to the development of obesity and to a wide number of comorbidities, such as insulin-resistance, cardiovascular disease, among others. In this scenario, it becomes imperative to develop new approaches to the treatment and prevention of obesity and its comorbidities. It has been documented that the browning of WAT could be a suitable strategy to tackle the obesity epidemic that is developing worldwide. Currently there is an intense search for bioactive compounds with anti-obesity properties, which present the particular ability to generate thermogenesis in the brown adipose tissue (BAT) or beige. The present study provide recent information of the bioactive nutritional compounds capable of inducing thermogenesis and therefore capable of generate positive effects on health.

Similar content being viewed by others
Abbreviations
- AC:
-
Adenylate Cyclase (AC)
- ALA:
-
Alpha-linolenic acid
- cAMP:
-
Cyclic adenosine monophosphate
- AR-β3:
-
β3 adrenergic receptor
- ARA:
-
Arachidonic acid
- ATF2:
-
Activating Transcription Factor 2
- BAT:
-
Brown Adipose Tissue
- BC:
-
β-carotene
- CNS:
-
Central Nervous System
- DHA:
-
Docosahexaenoic acid
- DIO2:
-
Type II iodothyronine deiodinase
- EPA:
-
Eicosapentaenoic acid
- EGCG:
-
Epigallocatechin gallate
- FX:
-
Fucoxanthin
- 18FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography combined with computed tomography
- LCPUFA:
-
Long chain polyunsaturated fatty acids
- MC4R:
-
Melanocortin-4 receptor
- NE:
-
Norepinephrine
- PGC1α:
-
Peroxisome proliferation-activation receptor and coactivator 1α
- PPARα:
-
Receptor activado por proliferador de peroxisoma α
- PRDM16:
-
PR domain zinc finger protein 16
- RA:
-
Retinoic acid
- Rald:
-
Retinaldehyde
- RMR:
-
Resting metabolic rate
- RXR:
-
Retinoid X Receptor
- SAs:
-
Alkaloids of synephrine
- TLQP-21:
-
Neuroendocrine VGF-derived peptide
- UCP-1:
-
Uncoupling protein 1
- VGF:
-
Nerve growth factor inducible
- WAT:
-
White Adipose Tissue
- WHO:
-
World Health Organization
References
Zulet MA, Puchau B, Navarro C, Marti A, Martinez JA. Inflammatory biomarkers: the link between obesity and associated pathologies. Nutr Hosp. 2007;22(5):511–27.
Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2017;134:62–70. https://doi.org/10.1016/j.biochi.2016.09.007.
Gomez-Hernandez A, Perdomo L, Escribano O, Benito M. Role of white adipose tissue in vascular complications due to obesity. Clin Investig Arterioscler. 2013;25(1):27–35. https://doi.org/10.1016/j.arteri.2012.11.003.
Contreras C, Nogueiras R, Dieguez C, Medina-Gomez G, Lopez M. Hypothalamus and thermogenesis: heating the BAT, browning the WAT. Mol Cell Endocrinol. 2016;438:107–15. https://doi.org/10.1016/j.mce.2016.08.002.
Khan MI, Anjum FM, Sohaib M, Sameen A. Tackling metabolic syndrome by functional foods. Rev Endocr Metab Disord. 2013;14(3):287–97. https://doi.org/10.1007/s11154-013-9270-8.
Montanari T, Poscic N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obes Rev. 2017;18(5):495–513. https://doi.org/10.1111/obr.12520.
Lorente-Cebrian S, Costa AG, Navas-Carretero S, Zabala M, Martinez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69(3):633–51. https://doi.org/10.1007/s13105-013-0265-4.
Guo H, Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord. 2015;16(1):1–13. https://doi.org/10.1007/s11154-014-9302-z.
Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9(2):191–200. https://doi.org/10.5114/aoms.2013.33181.
Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31:33–47. https://doi.org/10.1146/annurev-nutr-072610-145209.
Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. https://doi.org/10.1038/nrendo.2013.204.
Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293(2):E444–52. https://doi.org/10.1152/ajpendo.00691.2006.
Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104(11):4401–6. https://doi.org/10.1073/pnas.0610615104.
Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659–67. https://doi.org/10.1038/ncb2740.
Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108(1):143–8. https://doi.org/10.1073/pnas.1010929108.
Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24(7):3057–67.
Martinez-deMena R, Obregon MJ. Insulin increases the adrenergic stimulation of 5′ deiodinase activity and mRNA expression in rat brown adipocytes; role of MAPK and PI3K. J Mol Endocrinol. 2005;34(1):139–51. https://doi.org/10.1677/jme.1.01568.
Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013;1831(5):969–85. https://doi.org/10.1016/j.bbalip.2012.12.002.
Shi SY, Zhang W, Luk CT, Sivasubramaniyam T, Brunt JJ, Schroer SA, et al. JAK2 promotes brown adipose tissue function and is required for diet- and cold-induced thermogenesis in mice. Diabetologia. 2016;59(1):187–96. https://doi.org/10.1007/s00125-015-3786-2.
Boutant M, Joffraud M, Kulkarni SS, Garcia-Casarrubios E, Garcia-Roves PM, Ratajczak J, et al. SIRT1 enhances glucose tolerance by potentiating brown adipose tissue function. Mol Metab. 2015;4(2):118–31. https://doi.org/10.1016/j.molmet.2014.12.008.
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–39.
Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11(4):257–62. https://doi.org/10.1016/j.cmet.2010.03.005.
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150(3):620–32. https://doi.org/10.1016/j.cell.2012.06.027.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60. https://doi.org/10.1038/nature07813.
Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. https://doi.org/10.1073/pnas.0705070104.
Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwartzenburg C, Morrison CD, et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014;3(7):681–93. https://doi.org/10.1016/j.molmet.2014.07.008.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383–95.
Kakuma T, Wang ZW, Pan W, Unger RH, Zhou YT. Role of leptin in peroxisome proliferator-activated receptor gamma coactivator-1 expression. Endocrinology. 2000;141(12):4576–82. https://doi.org/10.1210/endo.141.12.7804.
Rachid TL, Penna-de-Carvalho A, Bringhenti I, Aguila MB, Mandarim-de-Lacerda CA, Souza-Mello V. PPAR-alpha agonist elicits metabolically active brown adipocytes and weight loss in diet-induced obese mice. Cell Biochem Funct. 2015;33(4):249–56. https://doi.org/10.1002/cbf.3111.
Bartolomucci A, La Corte G, Possenti R, Locatelli V, Rigamonti AE, Torsello A, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci U S A. 2006;103(39):14584–9. https://doi.org/10.1073/pnas.0606102103.
Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–38. https://doi.org/10.1016/j.cmet.2011.06.020.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. https://doi.org/10.1056/NEJMoa0808949.
Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53(3):577–84.
Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173(15):2369–89. https://doi.org/10.1111/bph.13514.
Nirengi S, Homma T, Inoue N, Sato H, Yoneshiro T, Matsushita M, et al. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J Biomed Opt. 2016;21(9):091305. https://doi.org/10.1117/1.JBO.21.9.091305.
Ang QY, Goh HJ, Cao Y, Li Y, Chan SP, Swain JL, et al. A new method of infrared thermography for quantification of brown adipose tissue activation in healthy adults (TACTICAL): a randomized trial. J Physiol Sci. 2017;67(3):395–406. https://doi.org/10.1007/s12576-016-0472-1.
Allison DB, Cutter G, Poehlman ET, Moore DR, Barnes S. Exactly which synephrine alkaloids does Citrus aurantium (bitter orange) contain? Int J Obes. 2005;29(4):443–6. https://doi.org/10.1038/sj.ijo.0802879.
Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011;25(10):1421–8. https://doi.org/10.1002/ptr.3490.
de Oliveira AL, Comar JF, de Sa-Nakanishi AB, Peralta RM, Bracht A. The action of p-synephrine on hepatic carbohydrate metabolism and respiration occurs via both ca(2+)-mobilization and cAMP production. Mol Cell Biochem. 2014;388(1–2):135–47. https://doi.org/10.1007/s11010-013-1905-2.
Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH, Chung D, et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol. 2012;166(7):1843–55. https://doi.org/10.1007/s12010-012-9602-2.
Woo MN, Jeon SM, Shin YC, Lee MK, Kang MA, Choi MS. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol Nutr Food Res. 2009;53(12):1603–11. https://doi.org/10.1002/mnfr.200900079.
Hashimoto T, Ozaki Y, Taminato M, Das SK, Mizuno M, Yoshimura K, et al. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr. 2009;102(2):242–8. https://doi.org/10.1017/S0007114508199007.
Bonet ML, Canas JA, Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112–25. https://doi.org/10.1016/j.abb.2015.02.022.
Serra F, Bonet ML, Puigserver P, Oliver J, Palou A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int J Obes Relat Metab Disord. 1999;23(6):650–5.
Guo H, Foncea R, O'Byrne SM, Jiang H, Zhang Y, Deis JA, et al. Lipocalin 2, a regulator of retinoid homeostasis and retinoid-mediated thermogenic activation in adipose tissue. J Biol Chem. 2016;291(21):11216–29. https://doi.org/10.1074/jbc.M115.711556.
Zhao M, Chen X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem Biophys Res Commun. 2014;450(4):1446–51. https://doi.org/10.1016/j.bbrc.2014.07.010.
Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr. 2000;84(2):175–84.
Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, et al. Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int J Obes Relat Metab Disord. 1997;21(11):955–62.
Fleckenstein-Elsen M, Dinnies D, Jelenik T, Roden M, Romacho T, Eckel J. Eicosapentaenoic acid and arachidonic acid differentially regulate adipogenesis, acquisition of a brite phenotype and mitochondrial function in primary human adipocytes. Mol Nutr Food Res. 2016;60(9):2065–75. https://doi.org/10.1002/mnfr.201500892.
Pisani DF, Ghandour RA, Beranger GE, Le Faouder P, Chambard JC, Giroud M, et al. The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol Metab. 2014;3(9):834–47. https://doi.org/10.1016/j.molmet.2014.09.003.
Laiglesia LM, Lorente-Cebrian S, Prieto-Hontoria PL, Fernandez-Galilea M, Ribeiro SM, Sainz N, et al. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J Nutr Biochem. 2016;37:76–82. https://doi.org/10.1016/j.jnutbio.2016.07.019.
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47. https://doi.org/10.1093/ajcn/79.5.727.
Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, et al. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res. 2013;55(4):416–23. https://doi.org/10.1111/jpi.12089.
Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999;70(6):1040–5. https://doi.org/10.1093/ajcn/70.6.1040.
Decorde K, Teissedre PL, Sutra T, Ventura E, Cristol JP, Rouanet JM. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol Nutr Food Res. 2009;53(5):659–66. https://doi.org/10.1002/mnfr.200800165.
Arias N, Pico C, Teresa Macarulla M, Oliver P, Miranda J, Palou A, et al. A combination of resveratrol and quercetin induces browning in white adipose tissue of rats fed an obesogenic diet. Obesity (Silver Spring). 2017;25(1):111–21. https://doi.org/10.1002/oby.21706.
Chang HC, Peng CH, Yeh DM, Kao ES, Wang CJ. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014;5(4):734–9. https://doi.org/10.1039/c3fo60495k.
Rangel-Huerta OD, Aguilera CM, Martin MV, Soto MJ, Rico MC, Vallejo F, et al. Normal or high polyphenol concentration in Orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J Nutr. 2015;145(8):1808–16. https://doi.org/10.3945/jn.115.213660.
Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63. https://doi.org/10.2337/db09-0482.
Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes. 2015;39(6):967–76. https://doi.org/10.1038/ijo.2015.23.
Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141(2):1530–5. https://doi.org/10.1016/j.foodchem.2013.03.085.
Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53(7):1503–10. https://doi.org/10.1007/s00394-014-0655-6.
Oi-Kano Y, Kawada T, Watanabe T, Koyama F, Watanabe K, Senbongi R, et al. Oleuropein, a phenolic compound in extra virgin olive oil, increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J Nutr Sci Vitaminol (Tokyo). 2008;54(5):363–70.
Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun. 2015;466(2):247–53. https://doi.org/10.1016/j.bbrc.2015.09.018.
Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem. 2016;27:193–202. https://doi.org/10.1016/j.jnutbio.2015.09.006.
Yamashita Y, Wang L, Wang L, Tanaka Y, Zhang T, Ashida H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014;5(10):2420–9. https://doi.org/10.1039/c4fo00095a.
Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22(1):1–7. https://doi.org/10.1016/j.jnutbio.2010.06.006.
Berube-Parent S, Pelletier C, Dore J, Tremblay A. Effects of encapsulated green tea and guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br J Nutr. 2005;94(3):432–6.
Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K, et al. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr. 2017;105(4):873–81. https://doi.org/10.3945/ajcn.116.144972.
Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78(1):78–84. https://doi.org/10.1016/j.bcp.2009.03.021.
Wang S, Huang Y, Xu H, Zhu Q, Lu H, Zhang M, et al. Oxidized tea polyphenols prevent lipid accumulation in liver and visceral white adipose tissue in rats. Eur J Nutr. 2017;56(6):2037–48. https://doi.org/10.1007/s00394-016-1241-x.
Most J, Timmers S, Warnke I, Jocken JW, van Boekschoten M, de Groot P, et al. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am J Clin Nutr. 2016;104(1):215–27. https://doi.org/10.3945/ajcn.115.122937.
Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011;59(6):2663–71. https://doi.org/10.1021/jf1043508.
Pajuelo D, Quesada H, Diaz S, Fernandez-Iglesias A, Arola-Arnal A, Blade C, et al. Chronic dietary supplementation of proanthocyanidins corrects the mitochondrial dysfunction of brown adipose tissue caused by diet-induced obesity in Wistar rats. Br J Nutr. 2012;107(2):170–8. https://doi.org/10.1017/S0007114511002728.
Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–83. https://doi.org/10.1136/gutjnl-2014-307142.
Moghe SS, Juma S, Imrhan V, Vijayagopal P. Effect of blueberry polyphenols on 3T3-F442A preadipocyte differentiation. J Med Food. 2012;15(5):448–52. https://doi.org/10.1089/jmf.2011.0234.
You Y, Yuan X, Lee HJ, Huang W, Jin W, Zhan J. Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis. Food Funct. 2015;6(2):401–8. https://doi.org/10.1039/c4fo00719k.
Ma S, Feng J, Zhang R, Chen J, Han D, Li X, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxidative Med Cell Longev. 2017;2017:4602715. https://doi.org/10.1155/2017/4602715.
Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48(1):409–16. https://doi.org/10.1016/j.fct.2009.10.030.
Funding
This study was funded by Fondecyt Project (1171550); CONICYT, Chile.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Concha, F., Prado, G., Quezada, J. et al. Nutritional and non-nutritional agents that stimulate white adipose tissue browning. Rev Endocr Metab Disord 20, 161–171 (2019). https://doi.org/10.1007/s11154-019-09495-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-019-09495-y