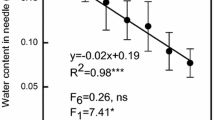
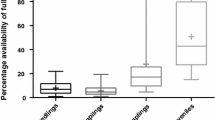
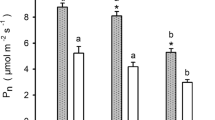
Abstract
To evaluate the acclimative ability of current-year and previous-year needles of a shade tolerant conifer Taxus baccata L. to contrasting irradiance conditions, seedlings were raised under 27% solar irradiance and at 3 years of age they were transferred to an experimental garden and grown for one season under full irradiance (HL), 18% irradiance (ML) or 5% irradiance (LL). Whereas previous year needles did not change anatomically, current year needles in HL were thicker and had a thicker palisade and spongy mesophyll, and greater leaf mass per area than ML or LL needles. LL needles had greater nitrogen concentration than HL needles irrespective of age but only previous year LL needles also had an increased N per area content, thanks to their lack of reduction in LMA. Adjustment of chlorophyll and carotenoid content occurred in both needle age classes with LL and ML needles having much higher concentrations but, in current year needles, only slightly higher per area content than HL needles. Chlorophyll a/b ratio was not affected by age or irradiance. These modifications had no significant effect on photosynthetic capacities, which did not significantly differ between the age classes in HL or LL treatment and between treatments. On the other hand, high growth irradiance resulted in a greater photochemical yield, photochemical quenching, apparent electron transport rate and inducible non-photochemical quenching in needles formed in the current season. In previous year needles, however, only inducible NPQ was enhanced by high irradiance with other parameters remaining identical among treatments. To test sensitivity to photoinhibition, at the end of the summer plants from the three irradiance levels were transferred to a HL situation and F v/F M was determined over the following 18 days. Sensitivity to photoinhibition was negatively related to growth irradiance and previous year needles were less photoinhibited than current year needles. Thus, differences in acclimation ability between needle age classes were most pronounced at the level of anatomy and light reactions of photosynthesis, both of which showed almost no plasticity in previous year needles but were considerably modified by irradiance in current year needles.








Similar content being viewed by others
Abbreviations
- ETR:
-
Apparent electron transport rate (μmol e− m−2 s−1)
- F V/F M :
-
Maximum quantum yield of photosystem II photochemistry
- F M :
-
Maximum fluorescence yield
- F M′:
-
Maximum fluorescence in the light
- F 0 :
-
Minimum fluorescence yield
- F S :
-
Steady-state fluorescence
- F 0′:
-
Minimum fluorescence yield in light-adapted state
- HL:
-
High light
- ML:
-
Medium light
- LL:
-
Low light
- NPQ:
-
Non-photochemical quenching of fluorescence
- qP:
-
Coefficient of photochemical quenching of chlorophyll fluorescence
- PPFD:
-
Photosynthetic photon flux density (μmol quanta m−2 s−1)
- PNUE:
-
Photosynthetic nitrogen use efficiency (μmol CO2 mol N−1 s−1)
- ΦPSII :
-
Quantum yield of PSII photochemistry
References
Adams WW III, Demmig-Adams B (1994) Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol Plant 92:451–458
Avalos G, Mulkey SS (1999). Photosynthetic acclimation of the liana Stigmaphyllon lindenianum to light changes in a tropical dry forest canopy. Oecologia 120:475–484
Bailey S, Horton P, Walters RG (2004) Acclimation of Arabidopsis thaliana to the light environment: the relationship between photosynthetic function and chloroplast composition. Planta 218:793–802
Barnes JD, Balaguer L, Manrique E et al (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ Exp Bot 32:85–100
Bauer H, Thoni W (1988) Photosynthetic acclimation in fully developed leaves of the juvenile and adult life phases of Hedera helix. Physiol Plant 73:31–37
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB et al (eds) Physiological plant ecology. I. Responses to the physical environment. Encyclopedia of Plant Physiology New Series, vol 12A. Springer, New York, pp 57–107
Brooks JR, Hinckley TM, Sprugel DG (1994) Acclimation responses of mature Abies amabilis sun foliage to shading. Oecologia 100:316–324
Canham CD, Denslow JS, Platt WJ et al (1990) Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can J For Res 20:620–631
Cornic G, Fresneau C (2002) Photosynthtetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann Bot 89:887–894
DeLucia HE, Day AT, Vogelmann CT (1992) Ultraviolet-B and visible light penetration into needles of two species of subalpine conifers during foliar development. Plant Cell Environ 15:921–929
Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39:474–482
Einhorn K, Rosenqvist E, Leverenz JW (2004) Photoinhibition in seedlings of Fraxinus and Fagus under natural light conditions: implications for forest regeneration? Oecologia 140:241–251
Eschrich W, Burchardt R, Essiamah S (1989) The induction of sun and shade leaves of the European beech (Fagus sylvatica L.): anatomical studies. Trees 3:1–10
Evans RJ (1993) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Aust J Plant Physiol 20:69–82
Ewers FW (1982) Secondary growth in needle leaves of Pinus longaeva (bristlecone pine) and other conifers: quantitative data. Am J Bot 69:1552–1559
Frak E, Le Roux X, Millard P et al (2001) Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ 24:1279–1288
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Givnish TJ (1988) Adaptation to sun and shade: a whole plant prospective. Aust J Plant Physiol 15:63–92
Grassi G, Bagnaresi U (2001) Foliar morphological and physiological plasticity in Picea abies and Abies alba saplings along a natural light gradient. Tree Physiol 21:959–967
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ 25:1021–1030
Havaux M, Kloppstech K (2001) The photoprotective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta 213:953–966
Hättenschwiler S (2001) Tree seedling growth in natural deep shade: functional traits related to interspecific variation in response to elevated CO2. Oecologia 129:31–42
Hendry GAF, Houghton JD, Brown SB (1987) The degradation of chlorophyll – a biological enigma. New Phytol 107:255–302
Kirchgeßner H-D, Reichert K, Hauff K et al (2003) Light and temperature, but not UV radiation, affect chlorophylls and carotenoids in Norway spruce needles (Picea abies (L.) Karst.). Plant Cell Environ 26:1169–1179
Lei TT, Lechowicz MJ (1998) Diverse responses of maple saplings to forest light regimes. Ann Bot 82:9–19
Lichtenthaler HK, Buschmann C, Döll M et al (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2:115–141
Makino A, Miyale C, Yokota A (2002) Physiological functions of water–water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43:1017–1026
Maxwell K, Johnson NG (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51:659–668
Meir P, Kruijt B, Broadmeadow M et al (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25:343–357
Mitchell AK (1998) Acclimation of Pacific yew (Taxus brevifolia) foliage to sun and shade. Tree Physiol 18:749–757
Murchie EH, Horton P (1997). Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448
Naidu SL, DeLucia EH (1998) Physiological and morphological acclimation of shade-grown tree seedlings to late-season canopy gap formation. Plant Ecol 138:27–40
Niinemets Ü (1997) Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Funct Ecol 11:518–531
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculations of qP and Fv′/Fm′ without measuring F 0 ′. Photosynth Res 54:135–142
Pearcy RW, Sims DA (1994) Photosynthetic acclimation to changing light environment. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants. Academic Press, New York, pp 145–174
Polle A (1996) Mehler reaction: friend or foe of photosynthesis. Bot Acta 109:84–89
Pons TL, de Jong-van Berkel YEM (2004) Species-specific variation in the importance of the spectral quality gradient in canopies as a signal for photosynthetic resource partitioning. Ann Bot 94:725–732
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 16:26–37
Reich PB, Ellsworth DS, Walters MB (1998) Leaf structure (specific leaf area) modulates photosynthesis–nitrogen relations: evidence from within and across species and functional groups. Funct Ecol 12:948–958
Robakowski P, Wyka T, Samardakiewicz S et al (2004) Growth, photosynthesis and needle structure of silver fir (Abies alba Mill.) seedlings under different canopies. For Ecol Manage 201:211–227
Sack L, Melcher PJ, Liu WH et al (2006) How strong is intracanopy leaf plasticity in temperate deciduous trees? Am J Bot 93:829–839
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am J Bot 79:449–455
Smillie RM, Hetherington SE (1999) Photoabatement by anthocyanin shields photosynthetic systems from light stress. Photosynthetica 36:451–463
Terashima I, Miyazawa SI, Hanba YT (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114:93–105
Thomas H (1997) Chlorophyll: a symptom and a regulator of plastid development. New Phytol 136:163–181
Thomas PA, Polwart A (2003) Taxus baccata L. J Ecol 91:489–524
Tognetti R, Minota G, Pinzauti S et al (1998) Acclimation to changing light conditions of long-term shade-grown beech (Fagus sylvatica L.) seedlings of different geographic origins. Trees 12:326–333
Tsuyama M, Shibata M, Kobayashi Y (2003) Leaf factors affecting the relationship between chlorophyll fluorescence and the rate of photosynthetic electron transport as determined from CO2 uptake. J Plant Physiol 160:1131–1139
Valladares F, Martiney-Ferri E, Balaguer L et al (2000) Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy. New Phytol 148:79–91
Vogelmann TC, Martin G (1993) The functional significance of palisade tissue: penetration of directional versus diffuse light. Plant Cell Environ 16:65–72
Warren CR, Adams MA (2004) Evergreen trees do not maximize instantaneous photosynthesis. Trends Plant Sci 9:270–274
Weston E, Thorogood K, Vinti G et al (2000) Light quality controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 211:807–815
Wyka TP, Robakowski P, Żytkowiak R (2007) Leaf acclimation to contrasting irradiance in juvenile evergreen and deciduous trees. Tree Physiol 27:1293–1306
Acknowledgements
We thank Mr. T. Szeszycki of Rokita Forest District for donating Taxus seedlings and Adam Mickiewicz University Botanical Garden for allowing the use of their growing space. The study was financed by Adam Mickiewicz University and August Cieszkowski Agricultural University Interdisciplinary Research Grant 512-00-056.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wyka, T., Robakowski, P. & Żytkowiak, R. Leaf age as a factor in anatomical and physiological acclimative responses of Taxus baccata L. needles to contrasting irradiance environments. Photosynth Res 95, 87–99 (2008). https://doi.org/10.1007/s11120-007-9238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9238-1