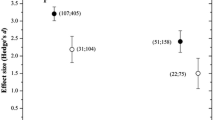
Abstract
Background and aims
Plant growth promoting rhizobacteria (PGPR) have been shown to reduce abiotic stress on plants, but these effects have not been quantitatively synthesized. We evaluated the degree to which plant growth promoting rhizobacteria (PGPR) improve plant performance with and without drought stress.
Methods
We used meta-analysis to summarize 52 published articles on the effects of PGPR on root mass, shoot mass and yield under well-watered and drought conditions. We also asked whether fertilization treatments, experimental conditions, inoculum taxonomic complexity, plant functional group, or inoculum delivery method introduce variation in the effect size of PGPR.
Results
Across all treatments, plants were highly responsive to PGPR; under well-watered conditions, root mass increased by 35%, shoot mass increased by 28%, and reproductive yield increased by 19%. Under drought conditions, the effect was even higher: root mass increased by 43%, shoot mass increased by 45%, and reproductive yield increased by 40%. The effect of PGPR was significantly larger under drought for shoot mass (p < 0.05) and reproductive yield (p < 0.05), but not for root mass. PGPR responsiveness also varied according to plant functional group, with C3 grass shoot production responding the least strongly to PGPR.
Conclusions
We demonstrate that PGPR are highly effective for improving plant growth, with a greater effect under drought for above ground traits. While previously known for their bio-control abilities, we show that PGPR may also contribute to drought amelioration and water conservation.





Similar content being viewed by others
References
References marked with an asterisk were included in the meta-analysis
Abramoff MD, Magalhães PJ, Ram SJ (2004) Image processing with image J. Biophoton Int 11:36–42
Adesemoye AO, Torbert HA, Kloepper JW (2009) Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol 58:921–929
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. King Saud Univ Sci 26:1–20
Angelini C, Griffin JN, van de Koppel J, Lamers LPM, Smolders AJP, Derksen-Hooijberg M, van der Heide T, Silliman BR (2016) A keystone mutualism underpins resilience of a coastal ecosystem to drought. Nat Commun 7:12473
Antoun H, Cj B, Goussard N, Chabot R, Lalande R (1998) Potential of rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
*Arvin P, Vafabakhsh J, Mazaheri D, Noormohamadi G, Azizi M (2012) Plant growth promoting rhizobacteria (PGPR) on yield, yield components and seed oil content of different cultivars and species of brassica oilseed rape. Ann Biol Res 9:4444–4451
*Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M (2011) Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J Microb Biotechnol 27:197–205
Augé RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570
*Bano Q, Ilyas N, Bano A, Zafar N, Akram A, Hassan F (2013) Effect of Azospirillum inoculation on maize (Zea mays L.) under drought stress. Pakistan J Bot 45:13–20
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2002) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
*Barman P, Singh SJ, Patel VB, Nain L, Pandey A (2015) Cleopatra mandarin (Citrus reshni Hort. Ex tan.) modulate physiological mechanisms to tolerate drought stress due to arbuscular mycorrhizal fungi and mycorrhizal helper bacteria. African J Microbiol Res 9:1236–1246
*Barnawal D, Maji D, Bharti N, Chanotiya CS, Kalra A (2013) ACC deaminase-containing Bacillus subtilis reduces stress ethylene-induced damage and improves mycorrhizal colonization and rhizobial nodulation in Trigonella foenum-graecum under drought stress. J Plant Growth Regul 32:809–822
Barriuso J, Solano BR, Lucas JA, Lobo AP, García-Villaraco A, Mañero FJG (2008) Ecology, genetic diversity and screening strategies of plant growth promoting rhizobacteria (PGPR). In: Ahmad I, Pichtel J, Hayat S (eds) Plantbacteria interactions: strategies and techniques to promote plant growth. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1–17
Bashan Y (1988) Nonspecific responses in plant growth, yield and root colonization of non cereal crop plants to inoculation with Azospirillum brasilense. Can J Bot 67:1317–1324
*Belimov AA, Dodd IC, Hontzeas N, Theobals JC, Safranova VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling. New Phytol 181:413–423
*Belimov AA, Dodd IC, Safronova VI, Shaposhnikov AI, Azaroza TS, Makarova NM, Davies WJ, Tikhonovich IA (2015) Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann Appl Biol 167:11–25
Bell TH, Cloutier-Hurteau B, Al-Otaibi F, Turmel M, Yergeau E, Courchesne F, St-Arnaud M (2015) Early rhizosphere microbiome composition is related to the growth and Zn uptake of willows introduced to a former landfill. Environ Microbiol 17:3025–3038
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Berríos G, Cabrera G, Gidekel M, Gutiérrez-Moraga A (2013) Characterization of a novel Antarctic plant growth-promoting bacterial strain and its interaction with Antarctic hair grass (Deschampsia Antarctica Desv). Polar Biol 36:349–362
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bianciotto V, Bonfante P (2002) Arbuscular mycorrhizal fungi: a specialised niche for rhizospheric and endocellular bacteria 81:365–371
Borenstein M, Hedges LV, Higgins J, Rothstein H (2009) Introduction to meta-analysis. Wiley, Hoboken, NJ
*Bresson J, Varoquaux F, Bontpart T, Touraine B, Vile D (2013) The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol 200:558–569
Burnett S, van Iersel M, Thomas P (2005) PEG alters morphology and nutrient composition of hydroponic impatiens. Hortic Sci 40:1768–1722
Calcagno V (2013) glmulti: Model selection and multimodel inference made easy. R package version 1.0.7. http://CRAN.R-project.org/package=glmulti
Carvalho FP (2006) Agriculture, pesticides, food security and food safety. Environ Sci Pol 9:685–692
Chandrasekaran M, Boughattas S, Hu S, Oh SH, Sa T (2014) A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 24:611–625
*Chaudhry V, Bhatia A, Bharti SK, Mishra SK, Chauhan PS, Mishra A, Sidhu OP, Nautiyal CS (2015) Metabolite profiling reveals abiotic stress tolerance in Tn5 mutant of Pseudomonas putida. PLoS One 10:e0113487
Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, De Noblet N, Friend AD, Friedlingstein P, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Papale D, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
*Cohen AC, Travaglia CN, Piccoli PN (2009) Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 87:455–462
*Cohen AC, Bottini R, Pontin M, Berli F, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN (2015) Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant 153:79–90
*Creus CM, Sueldo RJ, Barassi CA (2004) Water relations and yield in Azospirillum- inoculated wheat exposed to drought in the field. Can J Bot 82:273–281
*Dadrasan M, Chaichi MR, Pourbabae AA, Yazdani D, Keshavarz-Afshar R (2015) Deficit irrigation and biological fertilizer influence on yield and trigonelline production of fenugreek. Ind Crop Prod 77:156–162
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58
Dasilva EJ, Henriksson LE, Udris M (1977) Growth responses of mycorrhizal Boletus and Rhizopogon species to pesticides. Trans Br Mycol Soc 68:434–437
*Dastgiri L, Daneshian J, Rahmani HA, Sayfzadeh S (2013) Effect of PGPRs on agronomic characteristics of sunflower under different humidity regimes. Intl J Agron Plant Prod 4:2901–2905
*del Mar Alguacil M, Kohler J, Caravaca F, Roldán A (2009) Differential effects of Pseudomonas mendocina and Glomus intraradices on lettuce plants physiological response and aquaporin PIP2 gene expression under elevated atmospheric CO2 and drought. Microb Ecol 58:942–951
Egamberdieva D, Shrivastava M, Varma A (2015) Plant-growth-promoting rhizobacteria (PGPR) and medicinal plants. Springer, New York
Evenson RE, Gollin D (2003) Assessing the impact of the green revolution, 1960 to 2000. Science 300:758–762
Fan X, Hu H, Huang F, Li Y, Palta J (2015) Soil inoculation with Burkholderia sp. LD-11 has positive effect on water-use efficiency in inbred lines of maize. Plant Soil 390:337–349
*Fard MD, Habibi D, Fard FD (2011) Effect of plant growth promoting rhizobacteria and foliar application of amino acids and silicic acid on antioxidant enzyme activity of wheat under drought stress. Chem Eng 23:80–85
*Figueiredo MVB, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
*Gachomo EW, Kefela T, Houngnandan P, Baba-Moussa L, Kotchoni SO (2014) Bradyrhizobium japonicum IRAT FA3 increases biomass, yield and drought tolerance in plants. J Biosci (Z Naturforsch) 1:12–23
*Ghorbanpour M, Hatami M, Khavazi K (2013) Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloids production of Hyoscyamus niger under water deficit stress. Turk J Biol 37:350–360
Green RE, Cornell SJ, Scharlemann JPW, Balmford A (2005) Farming and the fate of wild nature. Science 307:550–555
*Habibi D (2014) The effects of plant growth-stimulating bacteria and foliar application of silicic acid and amino acids on biochemical markers in barley under drought stress. MAGNT Research Report 2:217–224
Hartmann AM, Schmid MD, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Hartmann M, Frey B, Mayer J, Mäder P, Widmer F (2015) Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9:1177–1194
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hester RE, Harrison RM (2005) Sustainability in agriculture. RSC Publishing, Cambridge, UK
Hetrick BAD, Gerscheksje K, Wilson GT (1988) Mycorrhizal dependence and growth habit of warm-season and cool-season tallgrass prairie plants. Can J Bot 66:1376–1380
Hetrick BAD, Wilson GWT, Todd TC (1990) Differential responses of C3 and C4 grasses to mycorrhizal symbiosis, phosphorus fertilization and soil microoganisms. Can J Bot 68:461–467
Hetrick BAD, Wilson GWT, Cox TS (1993) Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can J Bot 71:512–518
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umnamhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Jayne B, Quigley M (2014) Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: a meta-analysis. Mycorrhiza 24:109–119
*Joe MM, Kathikeyan B, Chauhan PS, Shagol C, Islam MR, Deiveekasundaram M, Sa T (2012) Survival of Azospirillum brasilense flocculated cells in alginate and its inoculation effect on growth and yield of maize under water deficit conditions. Eur J Soil Biol 50:198–206
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
*Khan N, Mishra A, Chauhan PS, Nautiyal CS (2011) Induction of Paenibacillus lentimorbus biofilm by sodium alginate and CaCl2 alleviates drought stress in chickpea. Ann Appl Biol 159:372–386
*Kheybari M, Daneshian J, Rahmani HA, Seyfzadeh S, Khiavi M (2013a) Response of sunflower head charachteristics to PGPR and amino acid application under water stress conditions. Intl J Agron Plant Prod 4:1760–1765
*Kheybari M, Daneshian J, Taei A, Hadi AR, Seyfzadeh S (2013b) Effect of PGPR and amino acid application on sunflower yield under different water deficit conditions. Int J Agric Res 3:467–471
Kiers ET, West SA, Denison RF (2002) Mediating mutualisms: farm management practices and evolutionary changes in symbiont co-operation. J Appl Ecol 39:745–754
Kiers ET, Hutton MG, Denison RF (2007) Human selection and the relaxation of legume defences against ineffective rhizobia. Proc Biol Sci 274:3119–3126
Kijne JW, Barker R, Molden DJ (2003) Water productivity in agriculture: limits and opportunities for improvement. CABI, IWMI, Wallingford, UK
Kivlin SN, Emery AM, Rudgers JA (2013) Fungal symbionts alter plant responses to global change. Am J Bot 100:1445–1457
Kloepper JK (1992) Plant growth-promoting rhizobacteria as biological control agents. In: Soil microbial ecology: applications in agricultural and environmental management. Ed. F B Metting. Taylor & Francis, Abingdon, pp 255–274
Kloepper JW, Schroth MN, Miller TD (1980a) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Ecol Epidemio 70:1078–1082
Kloepper JW, Schroth MN, Miller TD (1980b) Enhanced growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
*Kohler J, Hernández A, Caravaca F, Roldán A (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol 35:141–151
*Kohler J, Caravaca F, Roldán A (2009) Effect of drought on the stability of rhizosphere soil aggregates of Lactuva sativa grown in a degraded soil inoculated with PGPR and AM fungi. Soil Biol Biochem 42:429–434
*Kohler J, Knapp BA, Waldhuber S, Caravaca F, Roldán A, Insam H (2010) Effects of elevated CO2, water stress, and inoculation with Glomus intraradices or Pseudomonas mendocina on lettuce dry matter and rhizosphere microbial and functional diversity under growth chamber conditions. J Soils Sediments 10:1585–1597
Koricheva J, Gurevitch J, Mengersen K (2013) The handbook of meta-analysis in ecology and evolution. Princeton University Press, Princeton
Kumar S, Pandey P, Maheshwari DK (2009) Reduction in dose of chemical fertilizers and growth enhancement of sesame (Sesamum indicum L.) with application of rhizospheric competent Pseudomonas aeruginosa LES4. Eur J Soil Biol 45:334–340
*Kumar M, Mishra S, Dixit V, Agarwal L, Chauhan PS, Nautiyal S (2015) Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal Behav 11:e1071004
Lajeunesse MJ (2011) On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 92:2049–2055
Levine UY, Teal TK, Robertson GP, Schmidt TM (2011) Agriculture’s impact on microbial diversity and associated fluxes of carbon dioxide and methane. ISME J 5:1683–1691
*Liddycoat SM, Greenberg BM, Wolyn DJ (2009) The effect of plant growth-promoting rhizobacteria on asparagus seedlings and germinating seeds subjected to water stress under greenhouse conditions. Can J Microbiol 55:388–394
Lim J, Kim S (2013) Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol J 29:201–208
*Liu F, Xing S, Ma H, Du Z, Ma B (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97:9155–9164
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Malusà E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012 :12Article ID 491206
*Malusà E, Sala G, Chitarra W, Bardi L (2013) Improvement of response to low water availability in maize plants inoculated with selected rhizospheric microbial consortia under different irrigation regimes. J Environ Qual 12:13–21
Marasco R, Rolli E, Vigani G, Borin S, Sorlini C, Ouzari H, Zocchi G, Daffonchio D (2013) Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal Behav 8:e26741
Marulanda A, Barea JM, Azcón R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM Fungi and Bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124
*Miri MR, Moghadam HRT, Ghooshchi F, Zahedi H (2012) Growth, seed yield and phosphorus uptake of wheat as influenced by Azotobacter and arbuscular mycorrhizal colonization under drought stress conditions. Res Crop 13:21–28
*Moslemi Z, Habibi D, Asgharzadeh A, Ardakani MR, Modommadi A, Mohammadi M (2011) Response of phytohormones and biochemical markets of maize to super absorbent polymer and plant growth promoting rhizobacteria under drought stress. American-Eurasian J Agric & Environ Sci 10:787–796
*Moslemi Z, Habibi D, Asgharzadeh A, Ardakani MR, Mohammadi A, Sakari A (2012) Effects of super absorbent polymer and plant growth promoting rhizobacteria on yield and yield components of maize under drought stress and normal conditions. Am J Agric Environ Sci 12:358–364
Nakkeeran S, Dilantha Fernando WG, Siddiqui ZA (2005) Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In: Siddiqui (ed) PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 257–296
*Naseem H, Bano A (2014) Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Interact 9:689–701
*Naseri R, Moghadam A, Darabi F, Hatami A, Tahmasebei GRT (2013) The effect of deficit irrigation and Azotobacter chroococcum and Azospirillum brasilense on grain yield, yield components of maize (SC 704) as a second cropping in western Iran. Bull Env Pharmacol Life Sci 2:104–112
*Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Ngumbi E, Kloepper J (2016) Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol 105:109–125
*Omar MH, Zulkarami B, Ariffin N, Ismail MR, Saud HM, Amalina N, Habib SH, Kausar H (2014) Improved water use efficiency in rice under limited water environment through microbial inoculation. Intl J Food, Agric And Environ 12:149–154
Ortiz N, Armada E, Duque E, Roldán A, Azcón R (2015) Contribution of arbuscular myorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochtonous or allochthonous strains. J Plant Physiol 174:87–96
Pérez-Jaramillo JE, Mendes R, Raaijmakers JM (2016) Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol 90:635–644
Pringle EG, Akçay E, Raab TK, Dirzo R, Gordon DM (2013) Water stress strengthens mutualism among ants, trees, and scale insects. PLoS Biol 11:e1001705
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria URL http://www.Rproject.org
Requena N, Jimenez I, Toro M, Barea JM (1997) Interactions between plant growth-promothing rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in Mediterranean semi-arid ecosystems. New Phytol 136:667–677
Revillini D, Gehring CA, Johnson NC (2016) The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct Ecol 30:1086–1098
Rosenzweig C, Elliot J, Deryng D, Ruane AC, Müller C, Arneth A, Boote KJ, Folberth C, Glotter M, Khabarov N, Neumann K, Piontek F, Pugh TAM, Schmid E, Stehfest E, Yang H, Jones JW (2014) Assessing agricultural risks of climate change in the twenty-first century in a gridded crop model intercomparison. PNAS 111:3268–3273
Rudrappa T, Czymmek KJ, Pare PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556
*Ruíz-Sánchez M, Armada E, Muñoz Y, García de Salamone IE, Aroca R, Ruíz-Lozano JM, Azcón R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168:1031–1037
*Sadeghipour O, Abbasi S (2012) Soybean response to drought and seed inoculation. World Appl Sci J 17:55–60
Sahai S (1999) Biotechnology capacity of less developed countries in the Asian Pacific Rim. AgBioforum 2:189–197
*Sakari A, Ardakani MR, Khavazi K, Paknejad F, Moslemi Z (2012) Effect of Azospirillum lipoferum and Thiobacillus thioparus on quantitative and qualitative characters of rapeseed (Brassica napus L.) under water deficit conditions. Middle East J Sci Res 11:819–827
*Sandhya V, Ali SKZ, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Schmidhuber J, Tubiello FN (2007) Global food security under climate change. PNAS 104:19703–19708
*Shafighi A, Pazoki A, Asli DE (2014) Alleviation of water stress in fenugreek (Trigonella foenum-graecum L.) using different PGPR application methods. Adv Environ Biol 8:275–280
Shiklomanov IA, Rodda JC (2003) World water resources at the beginning of the twenty-first century. Cambridge Univ Press, Cambridge, UK, International Hydrology Series
*Shintu PV, Jayaram KM (2015) Phosphate solubilising bacteria (Bacillus polymyxa) - an effective approach to mitigate drought in tomato (Lycopersicon esculentum mill.). Tropical Plant Research 2:17–22
Shukla A, Kumar A, Jha A, Rao DVKN (2012) Phosphorus threshold for arbuscular mycorrhizal colonization of crops and tree seedlings. Biol Fertil Soils 48:109–116
*Singh NB, Singh D, Singh A (2015) Biological seed priming mitigates the effects of water stress in sunflower seedlings. Physiol Mol Biol Plants 21:207–214
Thomson BD, Robson AD, Abbott LK (1986) Effects of phosphorus on the formation of mycorrhizas by Gigaspora calospora and Glomus fasciculatum in relation to root carbohydrates. New Phytol 103:751–765
Timmusk S, Wagner EG (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant-Microbe Interact 12:951–959
Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. PLoS One 6:1–7
*Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Seisenbaeva G, Stenström E, Niinemets U (2014) Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLoS One 9:e96086
*Tittabutr P, Piromyou P, Longtonglang A, Noisa-Ngiam R, Boonkerd N, Teaumroong N (2013) Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci Plant Nutr 59:559–571
Toro M, Azcon R, Barea JM (1997) Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability and nutrient cycling. Appl Environ Microbiol 63:4408–4412
Treseder KK (2004) A meta-analysis of mycorrhizal response to nitrogen, phosphorus and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
van Groenigen KJ, Osenberg CW, Hungate BA (2011) Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216
Veresoglou SD, Menexes G (2010) Impact of inoculation with Azospirillum spp. on growth properties and seed yield of wheat: a meta-analysis of studies in the ISI web of science from 1981 to 2008. Plant Soil 337:469–480
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48 URL: http://www.jstatsoft.org/v36/i03/
*Volkmar KM, Bremer E (1998) Effects of seed inoculation with a strain of Pseudomonas fluorescens on root growth and activity of wheat in well-watered and drought-stressed glass-fronted rhizotrons. Can J Plant Sci 78:545–551
Vurukonda SSK, Vardharajula S, Shrivastava AS (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24
Waag CW, Jansa J, Schmid B, van der Heijden MGA (2011) Belowground biodiversity effects of plant symbionts supports aboveground productivity. Ecol Lett 14:1001–1009
Wada Y, Wisser D, Eisner S, Flörke M, Gerten D, Haddeland I, Hanasaki N, Masaki Y, Portmann FT, Stacke T, Tessler Z, Schewe J (2013) Multimodel projections and uncertainties of irrigation water demand under climate change. Geophys Res Lett 40:4626–4632
Wall DH, Moore JC (1999) Interactions underground: soil biodiversity, mutualism and ecosystem processes. Bioscience 49:109–117
Wang GL (2005) Agricultural drought in a future climate: results from 15 global climate models participating in the IPCC 4th assessment. Clim Dyn 25:739–753
Weese DJ, Heath KD, Dentinger BTM, Lau JA (2015) Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 69:631–642
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ (2006) A systematic review identified a lack of standardization in methods for handling missing variance data. J Clin Epidemiol 59:342–353
Wilson G, Harnett D (1997) Effects of mycorrhizae on plant growth and dynamics in experimental tall grass prairie microcosms. Am J Bot 84:478–482
Wilson GWT, Hartnett DC (1998) Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am J Bot 85:1732–1738
Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma 125:155–166
*Yasmin H, Bano A, Samiullah (2013) Screening of PGPR isolates from semi-arid region and their implication to alleviate drought stress. Pak J Bot 45:51–58
*Yuwono T, Handayani D, Soedarsono J (2005) The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust J Agric Res 56:715–721
*Zarmehri G, Moosavi SG, Zabihi HR, Seghateslami MJ (2013) The effect of plant growth promoting rhizobacteria (PGPR) and zinc fertilizer on forage yield of maize under water deficit stress conditions. TJEAS 23:3281–3290
Acknowledgements
We would like to acknowledge Dr. George Koch, Molly Shuman-Goodier, Brianna Finley and Elaine Pegoraro for assistance in reviewing this manuscript. Dr. Liza Holeski and Dr. Nathan Nieto provided conceptual feedback during the formulation of this study. We would also like to acknowledge three anonymous reviewers for their helpful critiques and suggestions. Funding to support Rachel Rubin was provided by NSF Dimensions of Biodiversity award no. 1241094, the ARCS Phoenix AZ Chapter, and the Soil Science Society of America (SSSA) Francis and Evelyn Clark Soil Biology Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Rubin, R.L., van Groenigen, K.J. & Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant Soil 416, 309–323 (2017). https://doi.org/10.1007/s11104-017-3199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3199-8