Abstract
Purpose
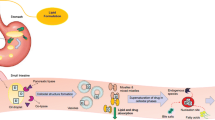
To evaluate the role of supersaturation in the in vivo absorption of fenofibrate (FFB), after oral administration in a medium-chain lipid-based formulation (MCLBF).
Methods
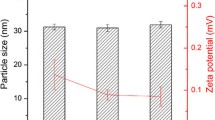
FFB was loaded at 90% and 20% w/w of saturated solubility in MCLBF. The two formulations were pre-dispersed in purified water at 5% w/w (ME90% and 20%, respectively) and orally administered to rats to measure in vivo luminal drug concentrations.
Results
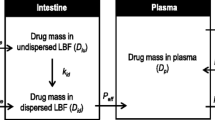
FFB precipitated in the stomach due to lipid digestion by gastric lipases and loss of solubilization capacity. This was most significant for ME90%. For ME90%, a high degree of supersaturation was also observed in the duodenum, however, precipitated FFB crystals rapidly re-dissolved. The combination of supersaturation and rapid re-dissolution appeared to drive effective absorption in the upper intestine. For ME20%, FFB precipitated in the stomach but not in the crystalline form and rapidly re-dissolved. Supersaturation in the duodenum again appeared to be the major driver of oral absorption.
Conclusions
The data provide one of the first studies of in vivo luminal drug concentration, supersaturation and absorption from lipid based formulations and suggests that for FFB, whilst very high supersaturation may drive in vitro and in vivo precipitation, re-dissolution and drug absorption is rapid and efficient.









Similar content being viewed by others
Abbreviations
- AP:
-
Aqueous phase
- 4-BPB:
-
4-bromophenylboronic acid
- CPLM:
-
Cross polarized light microscopy
- DMSO:
-
Dimethyl sulfoxide
- FFB:
-
Fenofibrate
- FFA:
-
Fenofibric acid
- FD-4:
-
Fluorescein isothiocyanate dextran 4000
- GI:
-
Gastrointestinal
- HPLC:
-
High performance liquid chromatography
- IS:
-
Internal standard
- LBF:
-
Lipid based formulation
- MCLBF:
-
Medium-chain lipid based formulation
- ME:
-
Microemulsion
- OP:
-
Oil phase
- PP:
-
Pellet phase
- PWSD:
-
Poorly water soluble drugs
- NaTC:
-
Sodium taurocholate
- AUC0-1440min :
-
The area under the plasma concentration–time curve from 0 to 1440 min
References
Feeney OM, Crum MF, McEvoy CL, Trevaskis NL, Williams HD, Pouton CW, et al. 50years of oral lipid-based formulations: provenance, progress and future perspectives. Adv Drug Deliv Rev. 2016;101:167–94.
Rezhdo O, Speciner L, Carrier R. Lipid-associated oral delivery: mechanisms and analysis of oral absorption enhancement. J Control Release. 2016;240:544–60.
Pandey V, Kohli S. Lipids and surfactants: the inside story of lipid-based drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2018;35:99–155.
Zaichik S, Steinbring C, Caliskan C, Bernkop-Schnürch A. Development and in vitro evaluation of a self-emulsifying drug delivery system (SEDDS) for oral vancomycin administration. Int J Pharm. 2019;554:125–33.
Yeap YY, Trevaskis NL, Quach T, Tso P, Charman WN, Porter CJH. Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol Pharm. 2013;10:1874–89.
Yeap YY, Trevaskis NL, Porter CJH. The potential for drug supersaturation during intestinal processing of lipid-based formulations may be enhanced for basic drugs. Mol Pharm. 2013;10:2601–15.
Yeap YY, Trevaskis NL, Porter CJH. Lipid absorption triggers drug supersaturation at the intestinal unstirred water layer and promotes drug absorption from mixed micelles. Pharm Res. 2013;30:3045–58.
Williams HD, Trevaskis NL, Yeap YY, Anby MU, Pouton CW, Porter CJH. Lipid-based formulations and drug supersaturation: harnessing the unique benefits of the lipid digestion/absorption pathway. Pharm Res. 2013;30:2976–92.
Anby MU, Williams HD, McIntosh M, Benameur H, Edwards GA, Pouton CW, et al. Lipid digestion as a trigger for supersaturation: evaluation of the impact of Supersaturation stabilization on the in vitro and in vivo performance of self-emulsifying drug delivery systems. Mol Pharm. 2012;9:2063–79.
Stillhart C, Imanidis G, Griffin BT, Kuentz M. Biopharmaceutical modeling of drug supersaturation during lipid-based formulation digestion considering an absorption sink. Pharm Res. 2014;31:3426–44.
Bevernage J, Forier T, Brouwers J, Tack J, Annaert P, Augustijns P. Excipient-mediated supersaturation stabilization in human intestinal fluids. Mol Pharm. 2011;8:564–70.
Gao P, Shi Y. Characterization of supersaturatable formulations for improved absorption of poorly soluble drugs. AAPS J. 2012;14:703–13.
Gao P, Akrami A, Alvarez F, Hu J, Li L, Ma C, et al. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J Pharm Sci. 2009;98:516–28.
Koyama H, Ito M, Terada K, Sugano K. Effect of seed particles on precipitation of weak base drugs in physiological intestinal conditions. Mol Pharm. 2016;13:2711–7.
Sugita M, Kataoka M, Sugihara M, Takeuchi S, Yamashita S. Effect of excipients on the particle size of precipitated pioglitazone in the gastrointestinal tract: impact on bioequivalence. AAPS J. 2014;16:1119–27.
Thomas N, Holm R, Garmer M, Karlsson JJ, Müllertz A, Rades T. Supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013;15:219–27.
Koziolek M, Carrière F, Porter CJH. Lipids in the stomach - implications for the evaluation of food effects on oral drug absorption. Pharm Res. 2018;35:55.
Pedersen PB, Vilmann P, Bar-Shalom D, Müllertz A, Baldursdottir S. Characterization of fasted human gastric fluid for relevant rheological parameters and gastric lipase activities. Eur J Pharm Biopharm. 2013;85:958–65.
Williams HD, Sassene P, Kleberg K, Bakala-N'Goma JC, Calderone M, Jannin V, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharm Sci. 2012;101:3360–80.
Crum MF, Trevaskis NL, Williams HD, Pouton CW, Porter CJH. A new in vitro lipid digestion - in vivo absorption model to evaluate the mechanisms of drug absorption from lipid-based formulations. Pharm Res. 2016;33:970–82.
Suys EJA, Chalmers DK, Pouton CW, Porter CJH. Polymeric precipitation inhibitors promote Fenofibrate Supersaturation and enhance drug absorption from a type IV lipid-based formulation. Mol Pharm. 2018;15:2355–71.
Keemink J, Mårtensson E, Bergström CAS. Lipolysis-permeation setup for simultaneous study of digestion and absorption in vitro. Mol Pharm. 2019;16:921–30.
Khan J, Rades T, Boyd BJ. Lipid-based formulations can enable the model poorly water-soluble weakly basic drug Cinnarizine to precipitate in an amorphous-salt form during in vitro digestion. Mol Pharm. 2016;13:3783–93.
Tanaka Y, Hara T, Waki R, Nagata S. Regional differences in the components of luminal water from rat gastrointestinal tract and comparison with other species. J Pharm Pharm Sci. 2012;15:510–8.
Sahbaz Y, Williams HD, Nguyen TH, Saunders J, Ford L, Charman SA, et al. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm. 2015;12:1980–91.
Weil A, Caldwell J, Strolin-Benedetti M. The metabolism and disposition of fenofibrate in rat, Guinea pig, and dog. Drug Metab Dispos. 1988;16:302–9.
Weil A, Caldwell J, Strolin-Benedetti M. The metabolism and disposition of 14C-fenofibrate in human volunteers. Drug Metab Dispos. 1990;18:115–20.
Williams HD, Sassene P, Kleberg K, Calderone M, Igonin A, Jule E, et al. LFCS consortium. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 3: understanding supersaturation versus precipitation potential during the in vitro digestion of type I, II, IIIA, IIIB and IV lipid-based formulations. Pharm Res. 2013;30:3059–76.
Devraj R, Williams HD, Warren DB, Porter CJH, Pouton CW. Choice of nonionic surfactant used to formulate type IIIA self-emulsifying drug delivery systems and the physicochemical properties of the drug have a pronounced influence on the degree of drug supersaturation that develops during in vitro digestion. J Pharm Sci. 2014;103:1050–63.
Dupont-Leclercq L, Giroux S, Henry B, Rubini P. Solubilization of amphiphilic carboxylic acids in nonionic micelles: determination of partition coefficients from pKa measurements and NMR experiments. Langmuir. 2007;23:10463–70.
Tanaka Y, Goto T, Kataoka M, Sakuma S, Yamashita S. Impact of luminal fluid volume on the drug absorption after oral administration: analysis based on in vivo drug concentration-time profile in the gastrointestinal tract. J Pharm Sci. 2015;104:3120–7.
Tanaka Y, Sugihara M, Kawakami A, Imai S, Itou T, Murase H, et al. In vivo analysis of supersaturation/precipitation/absorption behavior after oral administration of pioglitazone hydrochloride salt; determinant site of oral absorption. Eur J Pharm Sci. 2017;106:431–8.
Tanaka Y, Kawakami A, Nanimatsu A, Horio M, Matsuoka J, Wada T, et al. In vivo evaluation of supersaturation/precipitation/re-dissolution behavior of cinnarizine, a lipophilic weak base, in the gastrointestinal tract: the key process of oral absorption. Eur J Pharm Sci. 2017;96:464–71.
Kadono K, Yokoe J, Ogawara K, Higaki K, Kimura T. Analysis and prediction of absorption behavior for theophylline orally administered as powders based on gastrointestinal-transit-absorption (Gita) model. Drug Metab Pharmacokinet. 2002;17:307–15.
Takano R, Furumoto K, Shiraki K, Takata N, Hayashi Y, Aso Y, et al. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs; prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm Res. 2008;25:2334–44.
Bevernage J, Brouwers J, Annaert P, Augustijns P. Drug precipitation-permeation interplay: supersaturation in an absorptive environment. Eur J Pharm Biopharm. 2012;82:424–8.
Sassene PJ, Michaelsen MH, Mosgaard MD, Jensen MK, Van Den Broek E, Wasan KM, et al. In vivo precipitation of poorly soluble drugs from lipid-based drug delivery systems. Mol Pharm. 2016;13:3417–26.
Bakala-N'Goma JC, Williams HD, Sassene PJ, Kleberg K, Calderone M, Jannin V, et al. Toward the establishment of standardized in vitro tests for lipid-based formulations. 5. Lipolysis of representative formulations by gastric lipase. Pharm Res. 2015;32:1279–87.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–87.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and 'self-microemulsifying' drug delivery systems. Eur J Pharm Sci. 2000;11:S93–8.
Han SF, Yao TT, Zhang XX, Gan L, Zhu C, Yu HZ, et al. Lipid-based formulations to enhance oral bioavailability of the poorly water-soluble drug anethol trithione: effects of lipid composition and formulation. Int J Pharm. 2009;379:18–24.
Devraj R, Williams HD, Warren DB, Mohsin K, Porter CJH, Pouton CW. In vitro assessment of drug-free and fenofibrate-containing lipid formulations using dispersion and digestion testing gives detailed insights into the likely fate of formulations in the intestine. Eur J Pharm Sci. 2013;49:748–60.
Khan J, Hawley A, Rades T, Boyd BJ. In situ lipolysis and synchrotron small-angle X-ray scattering for the direct determination of the precipitation and solid-state form of a poorly WaterSoluble drug during digestion of a lipid-based formulation. J Pharm Sci. 2016;105:2631–9.
Sassene PJ, Knopp MM, Hesselkilde JZ, Koradia V, Larsen A, Rades T, et al. Precipitation of a poorly soluble model drug during in vitro lipolysis: characterization and dissolution of the precipitate. J Pharm Sci. 2010;99:4982–91.
Stillhart C, Imanidis G, Kuentz M. Insights into drug precipitation kinetics during in vitro digestion of a lipid-based drug delivery system using in-line raman spectroscopy and mathematical modeling. Pharm Res. 2013;30:3114–30.
Sassene PJ, Mosgaard MD, Löbmann K, Mu H, Larsen FH, Rades T, et al. Elucidating the molecular interactions occurring during drug precipitation of weak bases from lipid-based formulations: a case study with cinnarizine and a long chain self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12:4067–76.
Acknowledgments
This research was partially supported by the Nagai Foundation Tokyo research grant 2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sheng Qi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 769 kb)
Rights and permissions
About this article
Cite this article
Tanaka, Y., Tay, E., Nguyen, TH. et al. Quantifying In Vivo Luminal Drug Solubilization -Supersaturation-Precipitation Profiles to Explain the Performance of Lipid Based Formulations. Pharm Res 37, 47 (2020). https://doi.org/10.1007/s11095-020-2762-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-2762-9