Abstract
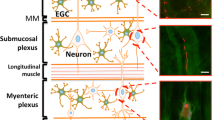
The pathogenesis of Parkinson’s disease (PD) involves the accumulation of aggregated forms of α-synuclein in the body. The location for the initiation of misfolded forms of α-synuclein is now a contentious issue, what was once thought to be a disease of the central nervous system (CNS) now appears to involve multiple organs in the body. In particular, the two regions in the body where the nervous system is exposed to the environment, the olfactory bulb and the enteric nervous system, are now thought to play an important role in the initial phase of the disease. Epidemiological studies point to the gastrointestinal tract, including the appendix, as a potential site for the misfolding and transmission of α-synuclein, with the vagus nerve providing a conduit between the gut and brain. A growing body of animal studies also support this pathway, implicating the transmission of pathological α-synuclein from outside the CNS in the development of PD.


Similar content being viewed by others
References
Feigin VL (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897
Tysnes O-B, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm 124(8):901–905
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272
Recasens A, Dehay B (2014) Alpha-synuclein spreading in Parkinson’s disease. Front Neuroanat 8:159
Recasens A et al (2014) Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75(3):351–362
Shahmoradian SH et al (2019) Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nat Neurosci 22(7):1099–1109
Braak H et al (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24(2):197–211
Pellicano C et al (2007) Prodromal non-motor symptoms of Parkinson's disease. Neuropsychiatr Dis Treat 3(1):145–152
Grey M et al (2011) Membrane Interaction of alpha-synuclein in different aggregation states. J Parkinsons Dis 1(4):359–371
Cole NB et al (2008) Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res 314(10):2076–2089
Nerius M, Doblhammer G, Tamguney G (2019) GI infections are associated with an increased risk of Parkinson's disease. Gut. https://doi.org/10.1136/gutjnl-2019-318822
Lin JC et al (2016) Association between Parkinson's disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm Bowel Dis 22(5):1049–1055
Villumsen M et al (2019) Inflammatory bowel disease increases the risk of Parkinson's disease: a Danish nationwide cohort study 1977–2014. Gut 68(1):18–24
Weimers P et al (2019) Inflammatory bowel disease and Parkinson's disease: a nationwide swedish cohort study. Inflamm Bowel Dis 25(1):111–123
Prusiner SB et al (1998) Prion protein biology. Cell 93(3):337–348
Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24:519–550
Hafner Bratkovic I (2017) Prions, prionoid complexes and amyloids: the bad, the good and something in between. Swiss Med Wkly 147:w14424
Brundin P, Ma JY, Kordower JH (2016) How strong is the evidence that Parkinson's disease is a prion disorder? Curr Opin Neurol 29(4):459–466
Purro SA et al (2018) Transmission of amyloid-β protein pathology from cadaveric pituitary growth hormone. Nature 564(7736):415–419
Giaccone G et al (2019) Iatrogenic early onset cerebral amyloid angiopathy 30 years after cerebral trauma with neurosurgery: vascular amyloid deposits are made up of both Aβ40 and Aβ42. Acta Neuropathol Commun 7(1):70–70
Acevedo-Morantes CY, Wille H (2014) The structure of human prions: from biology to structural models-considerations and pitfalls. Viruses 6(10):3875–3892
Krammer C et al (2009) The yeast Sup35NM domain propagates as a prion in mammalian cells. Proc Natl Acad Sci USA 106(2):462–467
Delenclos M et al (2017) Investigation of endocytic pathways for the internalization of exosome-associated oligomeric alpha-synuclein. Front Neurosci 11:172
Kim WS, Kagedal K, Halliday GM (2014) Alpha-synuclein biology in Lewy body diseases. Alzheimers Res Therapy 6(5):73
Theillet F-X et al (2016) Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 530:45
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47(3):e147–e147
Collier TJ et al (2016) Is alpha-synuclein loss-of-function a contributor to Parkinsonian pathology? Evidence from non-human primates. Front NeuroSci 10:12–12
Aulić S et al (2014) Defined α-synuclein prion-like molecular assemblies spreading in cell culture. BMC Neurosci 15:69–69
Tarutani A et al (2018) Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol Commun 6(1):29
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11(4):301–307
Hartmann A et al (2017) Exosomes and the prion protein: more than one truth. Front NeuroSci 11:194–194
Pezza JA, Serio TR (2007) Prion propagation the role of protein dynamics. Prion 1(1):36–43
Cheng L, Zhao WT, Hill AF (2018) Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol Aspects Med 60:62–68
Merrick WC, Hershey JW (1996) The pathway and mechanism of eukaryotic protein synthesis. Cold Spring Harbor Monogr Arch 30:31–69
Hawkes CH, Del K, Tredici, Braak H (2007) Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33(6):599–614
Makin S (2016) The prion principle. Nature 538(7626):S13–S16
Rietdijk CD et al (2017) Exploring Braak's hypothesis of Parkinson's disease. Front Neurol 8:37
Kordower JH et al (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 14(5):504–506
Li JY et al (2008) Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 14(5):501–503
Gao HM et al (2011) Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environ Health Perspect 119(6):807–814
Luk KC et al (2009) Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci 106(47):20051–20056
Ulusoy A et al (2017) Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol 133(3):381–393
Câmara R, Griessenauer CJ et al (2015) Chap. 27: anatomy of the vagus nerve. In: Tubbs RS et al (eds) Nerves and nerve injuries. Academic Press, San Diego, pp 385–397
Svensson E et al (2015) Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 78(4):522–529
Liu BJ et al (2017) Vagotomy and Parkinson disease a Swedish register-based matched-cohort study. Neurology 88(21):1996–2002
Luk KC et al (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338(6109):949–953
Holmqvist S et al (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128(6):805–820
Kim S et al (2019) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. https://doi.org/10.1016/j.neuron.2019.05.035
Van Den Berge N et al (2019) Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol 138:535–550
Lohmann S et al (2019) Oral and intravenous transmission of alpha-synuclein fibrils to mice. Acta Neuropathol 138:515–533
Ellis H, Mahadevan V (2014) Anatomy of the caecum, appendix and colon. Surgery (Oxford) 32(4):155–158
Girard-Madoux MJH et al (2018) The immunological functions of the appendix: an example of redundancy? Semin Immunol 36:31–44
Stokholm MG et al (2016) Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol 79(6):940–949
Killinger BA et al (2018) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aar5280
Stojkovska I, Wagner BM, Morrison BE (2015) Parkinson's disease and enhanced inflammatory response. Exp Biol Med (Maywood, N.J.) 240(11):1387–1395
Chen X, Guo C, Kong J (2012) Oxidative stress in neurodegenerative diseases. Neural Regen Res 7(5):376–385
Grey M et al (2015) Acceleration of α-synuclein aggregation by exosomes. J Biol Chem 290(5):2969–2982
Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474:307
Duricova D et al (2014) Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis 8(11):1351–1361
Head KA, Jurenka JS (2003) Inflammatory bowel disease Part 1: ulcerative colitis–pathophysiology and conventional and alternative treatment options. Altern Med Rev 8(3):247–283
Head K, Jurenka JS (2004) Inflammatory bowel disease. Part II: Crohn's disease—pathophysiology and conventional and alternative treatment options. Altern Med Rev 9(4):360–401
Weimers P et al (2019) Association between inflammatory bowel disease and Parkinson's disease: seek and you shall find? Gut 68(1):175–176
Villumsen M et al (2018) Authors’ response: Association between IBD and Parkinson’s disease: seek and you shall find? Gut 68(9):1722
Barrett JC et al (2008) Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40(8):955–962
Di Fonzo A et al (2006) Comprehensive analysis of the LRRK2 gene in sixty families with Parkinson's disease. Eur J Hum Genet 14(3):322–331
Wallings R, Manzoni C, Bandopadhyay R (2015) Cellular processes associated with LRRK2 function and dysfunction. FEBS J 282(15):2806–2826
Liu Z et al (2011) The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12(11):1063–1070
Cook DA et al (2017) LRRK2 levels in immune cells are increased in Parkinson's disease. NPJ Parkinsons Dis 3:11
Migheli R et al (2013) LRRK2 affects vesicle trafficking, neurotransmitter extracellular level and membrane receptor localization. PLoS ONE 8(10):e77198
Russo I, Bubacco L, Greggio E (2014) LRRK2 and neuroinflammation: partners in crime in Parkinson's disease? J Neuroinflammation 11:52
Loureiro ACCF, Barbosa LER (2018) Appendectomy and Crohn's disease. J Coloproctol. https://doi.org/10.1016/j.jcol.2017.12.004
Andersson RE et al (2003) Appendectomy is followed by increased risk of Crohn's disease. Gastroenterology 124(1):40–46
Sahami S et al (2016) The link between the appendix and ulcerative colitis: clinical relevance and potential immunological mechanisms. Am J Gastroenterol 111(2):163–169
Kaplan GG et al (2007) The risk of developing Crohn's disease after an appendectomy: a population-based cohort study in Sweden and Denmark. Gut 56(10):1387–1392
Radford-Smith GL et al (2002) Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut 51(6):808–813
Kalaitzakis ME et al (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of α-synuclein staging. Neuropathol Appl Neurobiol 34(3):284–295
Burke RE, Dauer WT, Vonsattel JP (2008) A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol 64(5):485–491
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18(2):101–113
EL-AGNAF OMA et al (2003) α-Synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17(13):1945–1947
Stuendl A et al (2016) Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson's disease and dementia with Lewy bodies. Brain 139:481–494
Mathieu M et al (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21(1):9–17
Abounit S et al (2016) Tunneling nanotubes spread fibrillar alpha-synuclein by intercellular trafficking of lysosomes. EMBO J 35(19):2120–2138
Matsumoto A et al (2017) Role of Phosphatidylserine-Derived Negative Surface Charges in the Recognition and Uptake of Intravenously Injected B16BL6-Derived Exosomes by Macrophages. J Pharm Sci 106(1):168–175
Aspelund A et al (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212(7):991–999
Da Mesquita S et al (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560(7717):185–191
Ahn JH et al (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572:62–66
Braak H et al (2003) Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110(5):517–536
Brooks PL et al (2012) Reconciling Braak's model of Parkinson's disease with a prion-like spread of alpha synuclein pathology. Basal Ganglia 2(4):167–170
Schlicke CP (1963) Complications of vagotomy. Am J Surg 106(2):206–216
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazurskyy, A., Howitt, J. Initiation and Transmission of α-Synuclein Pathology in Parkinson’s Disease. Neurochem Res 44, 2685–2694 (2019). https://doi.org/10.1007/s11064-019-02896-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02896-0