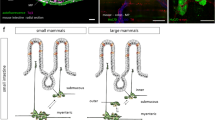
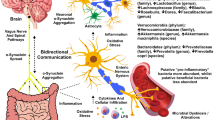
Abstract
The classic view portrays Parkinson disease (PD) as a motor disorder resulting from loss of substantia nigra pars compacta dopaminergic neurons. Multiple studies, however, describe prodromal, non-motor dysfunctions that affect the quality of life of patients who subsequently develop PD. These prodromal dysfunctions comprise a wide array of gastrointestinal motility disorders including dysphagia, delayed gastric emptying and chronic constipation. The histological hallmark of PD — misfolded α-synuclein aggregates that form Lewy bodies and neurites — is detected in the enteric nervous system prior to clinical diagnosis, suggesting that the gastrointestinal tract and its neural (vagal) connection to the central nervous system could have a major role in disease aetiology. This Review provides novel insights on the pathogenesis of PD, including gut-to-brain trafficking of α-synuclein as well as the newly discovered nigro–vagal pathway, and highlights how vagal connections from the gut could be the conduit by which ingested environmental pathogens enter the central nervous system and ultimately induce, or accelerate, PD progression. The pathogenic potential of various environmental neurotoxicants and the suitability and translational potential of experimental animal models of PD will be highlighted and appraised. Finally, the clinical manifestations of gastrointestinal involvement in PD and medications will be discussed briefly.
Key points
-
Gastrointestinal dysfunction, including dysphagia, delayed gastric emptying and constipation, can be detected up to 20 years prior to Parkinson disease (PD) diagnosis.
-
Lack of understanding of the mechanisms and pathophysiology hamper the diagnosis and clinical treatment of PD-related gastrointestinal dysfunction.
-
Both a ‘bottom-up’ and ‘top-down’ aetiology of PD have been proposed; experimental evidence suggests that these hypotheses are not mutually exclusive.
-
Experimental as well as clinical data suggest that PD is more a circuit-restricted than a cell-restricted disease.


Similar content being viewed by others
References
Goedert, M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349, 1255555 (2015).
Cersosimo, M. G. & Benarroch, E. E. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov. Disord. 23, 1065–1075 (2008).
Cersosimo, M. G. et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J. Neurol. 260, 1332–1338 (2013).
Goedert, M., Spillantini, M. G., Del, T. K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
Hunn, B. H., Cragg, S. J., Bolam, J. P., Spillantini, M. G. & Wade-Martins, R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 38, 178–188 (2015).
Braak, H. & Del, T. K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv. Anat. Embryol. Cell Biol. 201, 1–119 (2009).
Herva, M. E. & Spillantini, M. G. Parkinson’s disease as a member of prion-like disorders. Virus Res. 207, 38–46 (2014).
Malek, N. et al. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease — a systematic review. Acta Neurol. Scand. 130, 59–72 (2014).
Del Tredici, K. & Braak, H. Review: sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol. Appl. Neurobiol. 42, 33–50 (2016).
Li, J. Y. et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008).
Kordower, J. H., Chu, Y., Hauser, R. A., Freeman, T. B. & Olanow, C. W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14, 504–506 (2008).
Hansen, C. et al. Alpha-synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 121, 715–725 (2011).
Angot, E. et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS ONE 7, e39465 (2012).
Desplats, P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009).
Steiner, J. A., Quansah, E. & Brundin, P. The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res. 373, 161–173 (2018).
Borghammer, P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov. Disord. 33, 48–57 (2018).
Brundin, P. & Melki, R. Prying into the prion hypothesis for Parkinson’s disease. J. Neurosci. 37, 9808–9818 (2017).
Surmeier, D. J., Obeso, J. A. & Halliday, G. M. Parkinson’s disease is not simply a prion disorder. J. Neurosci. 37, 9799–9807 (2017).
Volpicelli-Daley, L. A. et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Fasano, A., Visanji, N. P., Liu, L. W., Lang, A. E. & Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639 (2015).
Cersosimo, M. G. & Benarroch, E. E. Central control of autonomic function and involvement in neurodegenerative disorders. Handb. Clin. Neurol. 117, 45–57 (2013).
Jost, W. H. Gastrointestinal dysfunction in Parkinson’s disease. J. Neurol. Sci. 289, 69–73 (2010).
Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 15 (Suppl. 1), 14–20 (2008).
Pfeiffer, R. F. Gastrointestinal dysfunction in Parkinson’s disease. Curr. Treat. Options Neurol. 20, 54 (2018).
Liddle, R. A. Parkinson’s disease from the gut. Brain Res. 1693, 201–206 (2018).
Schapira, A. H. V., Chaudhuri, K. R. & Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 509 (2017).
Abbott, R. D. et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–462 (2001).
Hilton, D. et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 127, 235–241 (2014).
Iljina, M. et al. Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. Proc. Natl Acad. Sci. USA 113, E1206–E1215 (2016).
Cersosimo, M. G. & Benarroch, E. E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 46, 559–564 (2012).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Hawkes, C. H., Del Tredici, K. & Braak, H. A timeline for Parkinson’s disease. Parkinsonism Relat. Disord. 16, 79–84 (2010).
Klingelhoefer, L. & Reichmann, H. Pathogenesis of Parkinson disease — the gut–brain axis and environmental factors. Nat. Rev. Neurol. 11, 625–636 (2015).
Bottner, M. et al. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol. Dis. 48, 474–480 (2012).
Shin, C. et al. Fundamental limit of alpha-synuclein pathology in gastrointestinal biopsy as a pathologic biomarker of Parkinson’s disease: comparison with surgical specimens. Parkinsonism Relat. Disord. 44, 73–78 (2017).
Barrenschee, M. et al. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun. 5, 1 (2017).
Visanji, N. P., Brooks, P. L., Hazrati, L. N. & Lang, A. E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 1, 2 (2013).
Dunning, C. J., George, S. & Brundin, P. What’s to like about the prion-like hypothesis for the spreading of aggregated alpha-synuclein in Parkinson disease? Prion 7, 92–97 (2013).
Goedert, M., Clavaguera, F. & Tolnay, M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325 (2010).
Olanow, C. W. & Brundin, P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov. Disord. 28, 31–40 (2013).
Angot, E., Steiner, J. A., Hansen, C., Li, J. Y. & Brundin, P. Are synucleinopathies prion-like disorders? Lancet Neurol. 9, 1128–1138 (2010).
Anselmi, L., Toti, L., Bove, C., Hampton, J. & Travagli, R. A. A nigro–vagal pathway controls gastric motility and is affected in a rat model of parkinsonism. Gastroenterology 153, 1581–1593 (2017).
Anselmi, L. et al. Ingestion of subthreshold doses of environmental toxins induces ascending parkinsonism in the rat. Naturepj Parkinson’s Dis. 4, 30 (2018).
Svensson, E. et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol 78, 522–529 (2015).
Tysnes, O. B. et al. Does vagotomy reduce the risk of Parkinson’s disease? Ann. Neurol. 78, 1011–1012 (2015).
Lionnet, A. et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 135, 1–12 (2018).
Attems, J. & Jellinger, K. A. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol. Appl. Neurobiol. 34, 466–467 (2008).
Kalaitzakis, M. E., Graeber, M. B., Gentleman, S. M. & Pearce, R. K. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease: a critical analysis of alpha-synuclein staging. Neuropathol. Appl. Neurobiol. 34, 284–295 (2008).
Ulusoy, A. et al. Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 133, 381–393 (2017).
Chandra, R., Hiniker, A., Kuo, Y. M., Nussbaum, R. L. & Liddle, R. A. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2, e92295 (2017).
Kim, S. et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641 (2019).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820 (2014).
McDowell, K. & Chesselet, M. F. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 46, 597–606 (2012).
Cannon, J. R. & Greenamyre, J. T. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog. Brain Res. 184, 17–33 (2010).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3, 1301–1306 (2000).
Drolet, R. E., Cannon, J. R., Montero, L. & Greenamyre, J. T. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol. Dis. 36, 96–102 (2009).
Greene, J. G., Noorian, A. R. & Srinivasan, S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp. Neurol. 218, 154–161 (2009).
Tasselli, M. et al. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol. Motil. 25, e183–e193 (2013).
Pan-Montojo, F. et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2, 898 (2012).
Elbaz, A. et al. Professional exposure to pesticides and Parkinson disease. Ann. Neurol. 66, 494–504 (2009).
Koller, W. C. Paraquat and Parkinson’s disease. Neurology 36, 1147 (1986).
Liou, H. H. et al. Environmental risk factors and Parkinson’s disease: a case–control study in Taiwan. Neurology 48, 1583–1588 (1997).
Betarbet, R., Sherer, T. B. & Greenamyre, J. T. Animal models of Parkinson’s disease. Bioessays 24, 308–318 (2002).
Rudyk, C., Litteljohn, D., Syed, S., Dwyer, Z. & Hayley, S. Paraquat and psychological stressor interactions as pertains to parkinsonian co-morbidity. Neurobiol. Stress. 2, 85–93 (2015).
Naudet, N. et al. Oral exposure to paraquat triggers earlier expression of phosphorylated alpha-synuclein in the enteric nervous system of A53T mutant human alpha-synuclein transgenic mice. J. Neuropathol. Exp. Neurol. 76, 1046–1057 (2017).
Bove, C., Coleman, F. H. & Travagli, R. A. Characterization of the basic membrane properties of neurons of the rat dorsal motor nucleus of the vagus in paraquat-induced models of parkinsonism. Neuroscience 418, 122–132 (2019).
Bove, C., Anselmi, L. & Travagli, R. A. Altered gastric tone and motility response to brainstem dopamine in a rat model of parkinsonism. Am. J. Physiol. Gastrointest. Liver Physiol 317, G1–G7 (2019).
Braak, H., Rub, U., Gai, W. P. & Del Tredici, K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110, 517–536 (2003).
Anderson, G. et al. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 207, 4–12 (2007).
Tian, Y. M. et al. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson’s disease. Neuroscience 153, 634–644 (2008).
Natale, G. et al. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 1355, 195–206 (2010).
Chaumette, T. et al. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental parkinsonism. Neurogastroenterol. Motil. 21, 215–222 (2009).
Ungerstedt, U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 5, 107–110 (1968).
Blandini, F. et al. Functional and neurochemical changes of the gastrointestinal tract in a rodent model of Parkinson’s disease. Neurosci. Lett. 467, 203–207 (2009).
Colucci, M. et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton. Neurosci. 169, 77–86 (2012).
Vegezzi, G. et al. Radiological analysis of gastrointestinal dysmotility in a model of central nervous dopaminergic degeneration: comparative study with conventional in vivo techniques in the rat. J. Pharmacol. Toxicol. Methods 70, 163–169 (2014).
Karasawa, H. et al. New ghrelin agonist, HM01, alleviates constipation and l-dopa-delayed gastric emptying in 6-hydroxydopamine rat model of Parkinson’s disease. Neurogastroenterol. Motil. 26, 1771–1782 (2014).
Zheng, L. F. et al. Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res. 1420, 59–67 (2011).
Toti, L. & Travagli, R. A. Gastric dysregulation induced by microinjection of 6-OHDA in the substantia nigra pars compacta of rats is determined by alterations in the brain–gut axis. Am. J. Physiol. Gastrointest. Liver Physiol 307, G1013–G1023 (2014).
Garrido-Gil, P., Rodriguez-Perez, A. I., Dominguez-Meijide, A., Guerra, M. J. & Labandeira-Garcia, J. L. Bidirectional neural interaction between central dopaminergic and gut lesions in Parkinson’s disease models. Mol. Neurobiol. 55, 7297–7316 (2018).
Levandis, G. et al. Response of colonic motility to dopaminergic stimulation is subverted in rats with nigrostriatal lesion: relevance to gastrointestinal dysfunctions in Parkinson’s disease. Neurogastroenterol. Motil. 27, 1783–1795 (2015).
Pellegrini, C. et al. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J. Neuroinflammation 13, 146 (2016).
Zheng, L. F. et al. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol. 211, 434–446 (2014).
Cannon, J. R. & Greenamyre, J. T. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci. 124, 225–250 (2011).
Hawkes, C. H., Del, T. K. & Braak, H. Parkinson’s disease: the dual hit theory revisited. Ann. NY Acad. Sci. 1170, 615–622 (2009).
Schapira, A. H. & Tolosa, E. Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat. Rev. Neurol. 6, 309–317 (2010).
Sulzer, D. & Surmeier, D. J. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov. Disord. 28, 715–724 (2013).
Conway, K. A., Harper, J. D. & Lansbury, P. T. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320 (1998).
Kuo, Y. M. et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 19, 1633–1650 (2010).
Noorian, A. R. et al. Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol. Dis. 48, 9–19 (2012).
Rockenstein, E. et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 68, 568–578 (2002).
Wang, L. et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol. Motil. 24, e425–e436 (2012).
Wang, L., Fleming, S. M., Chesselet, M. F. & Tache, Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 19, 873–876 (2008).
Hallett, P. J., McLean, J. R., Kartunen, A., Langston, J. W. & Isacson, O. Alpha-synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol. Dis. 47, 258–267 (2012).
Manfredsson, F. P. et al. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiol. Dis. 112, 106–118 (2018).
Travagli, R. A. & Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 13, 389–401 (2016).
Baintner, K., Jakab, G., Gyori, Z. & Kiss, P. Binding of FITC-labelled lectins to the gastrointestinal epithelium of the rat. Pathol. Oncol. Res. 6, 179–183 (2000).
Hind, A. et al. Primary afferent neurons intrinsic to the guinea-pig intestine, like primary afferent neurons of spinal and cranial sensory ganglia, bind the lectin, IB4. Cell Tissue Res. 321, 151–157 (2005).
Rudiger, H. & Gabius, H. J. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj. J. 18, 589–613 (2001).
Trojanowski, J. Q., Gonatas, J. O. & Gonatas, N. K. A light and electron microscopic study of the intraneuronal transport of horseradish peroxidase and wheat germ agglutinin-peroxidase conjugates in the rat visual system. J. Neurocytol. 10, 441–456 (1981).
Wan, X. C., Trojanowski, J. Q. & Gonatas, J. O. Cholera toxin and wheat germ agglutinin conjugates as neuroanatomical probes: their uptake and clearance, transganglionic and retrograde transport and sensitivity. Brain Res. 243, 215–224 (1982).
Thacker, M., Zhang, F. L., Jungnickel, S. R. & Furness, J. B. Binding of isolectin IB4 to neurons of the mouse enteric nervous system. J. Mol. Histol. 37, 61–68 (2006).
Mancheno, J. M., Tateno, H., Goldstein, I. J., Martinez-Ripoll, M. & Hermoso, J. A. Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 280, 17251–17259 (2005).
Zheng, J. et al. Dietary plant lectins appear to Bbe transported from the gut to gain access to and alter dopaminergic neurons of Caenorhabditis elegans, a potential etiology of Parkinson’s disease. Front. Nutr. 3, 7 (2016).
Gajbhiye, V. & Gong, S. Lectin functionalized nanocarriers for gene delivery. Biotechnol. Adv. 31, 552–562 (2013).
Lehr, C. M. & Gabor, F. Lectins and glycoconjugates in drug delivery and targeting. Adv. Drug Deliv. Rev. 56, 419–420 (2004).
Lindberg, I. et al. Chaperones in neurodegeneration. J. Neurosci. 35, 13853–13859 (2015).
Takada, A. et al. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78, 2943–2947 (2004).
Ho, S. C., Woo, J. & Lee, C. M. Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology 39, 1314–1318 (1989).
Scheider, W. L. et al. Dietary antioxidants and other dietary factors in the etiology of Parkinson’s disease. Mov. Disord. 12, 190–196 (1997).
Ogawa, H. & Date, K. The “white kidney bean incident” in Japan. Methods Mol. Biol. 1200, 39–45 (2014).
Rybner, C. et al. The cellular prion protein: a new partner of the lectin CBP70 in the nucleus of NB4 human promyelocytic leukemia cells. J. Cell Biochem. 84, 408–419 (2002).
Kalf, J. G., de Swart, B. J., Borm, G. F., Bloem, B. R. & Munneke, M. Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J. Neurol. 256, 1391–1396 (2009).
van Wamelen, D. J. et al. Drooling in Parkinson’s disease: prevalence and progression from the non-motor international longitudinal study. Dysphagia https://doi.org/10.1007/s00455-020-10102-5 (2020).
Beach, T. G. et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119, 689–702 (2010).
Cersosimo, M. G. et al. Hyposialorrhea as an early manifestation of Parkinson disease. Auton. Neurosci. 150, 150–151 (2009).
Perez Lloret, S. et al. Validation of a new scale for the evaluation of sialorrhea in patients with Parkinson’s disease. Mov. Disord. 22, 107–111 (2007).
Sung, H. Y., Park, J. W. & Kim, J. S. The frequency and severity of gastrointestinal symptoms in patients with early Parkinson’s disease. J. Mov. Disord. 7, 7–12 (2014).
Ma, K. et al. Weight loss and malnutrition in patients with Parkinson’s disease: current knowledge and future prospects. Front. Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00001 (2018).
Su, A., Gandhy, R., Barlow, C. & Triadafilopoulos, G. Clinical and manometric characteristics of patients with Parkinson’s disease and esophageal symptoms. Dis. Esophagus 30, 1–6 (2017).
Jones, C. A. et al. Identification of swallowing disorders in early and mid-stage Parkinson’s disease using pattern recognition of pharyngeal high-resolution manometry data. Neurogastroenterol. Motil. 30, e13236 (2018).
van Hooren, M. R., Baijens, L. W., Voskuilen, S., Oosterloo, M. & Kremer, B. Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 20, 800–807 (2014).
Su, A., Gandhy, R., Barlow, C. & Triadafilopoulos, G. A practical review of gastrointestinal manifestations in Parkinson’s disease. Parkinsonism Relat. Disord. 39, 17–26 (2017).
Evans, M. A. et al. Gastric emptying rate and the systemic availability of levodopa in the elderly parkinsonian patient. Neurology 31, 1288–1294 (1981).
Arai, E. et al. Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain 135, 1478–1485 (2012).
Vijayvargiya, P. et al. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 156, 1650–1660 (2019).
Knudsen, K., Szwebs, M., Hansen, A. K. & Borghammer, P. Gastric emptying in Parkinson’s disease — a mini-review. Parkinsonism Relat. Disord. 55, 18–25 (2018).
Bestetti, A., Capozza, A., Lacerenza, M., Manfredi, L. & Mancini, F. Delayed gastric emptying in advanced Parkinson disease: correlation with therapeutic doses. Clin. Nucl. Med. 42, 83–87 (2017).
Tarsy, D., Parkes, J. D. & Marsden, C. D. Metoclopramide and pimozide in Parkinson’s disease and levodopa-induced dyskinesias. J. Neurol. Neurosurg. Psychiatry 38, 331–335 (1975).
Simeonova, M. et al. Increased risk of all-cause mortality associated with domperidone use in Parkinson’s patients: a population-based cohort study in the UK. Br. J. Clin. Pharmacol. 84, 2551–2561 (2018).
Renoux, C. et al. Ventricular tachyarrhythmia and sudden cardiac death with domperidone use in Parkinson’s disease. Br. J. Clin. Pharmacol. 82, 461–472 (2016).
De Pablo-Fernandez, E., Passananti, V., Zarate-Lopez, N., Emmanuel, A. & Warner, T. Colonic transit, high-resolution anorectal manometry and MRI defecography study of constipation in Parkinson’s disease. Parkinsonism Relat. Disord. 66, 195–201 (2019).
Kupsky, W. J., Grimes, M. M., Sweeting, J., Bertsch, R. & Cote, L. J. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology 37, 1253–1255 (1987).
Mathers, S. E. et al. Anal sphincter dysfunction in Parkinson’s disease. Arch. Neurol. 46, 1061–1064 (1989).
Edwards, L. L., Quigley, E. M., Harned, R. K., Hofman, R. & Pfeiffer, R. F. Characterization of swallowing and defecation in Parkinson’s disease. Am. J. Gastroenterol. 89, 15–25 (1994).
Nullens, S. et al. Regional colon transit in patients with dys-synergic defaecation or slow transit in patients with constipation. Gut 61, 1132–1139 (2012).
Krogh, K., Ostergaard, K., Sabroe, S. & Laurberg, S. Clinical aspects of bowel symptoms in Parkinson’s disease. Acta Neurol. Scand. 117, 60–64 (2008).
Singaram, C. et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 346, 861–864 (1995).
Qualman, S. J., Haupt, H. M., Yang, P. & Hamilton, S. R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 87, 848–856 (1984).
Knudsen, K. et al. Gastrointestinal transit time in Parkinson’s disease using a magnetic tracking system. J. Parkinsons Dis. 7, 471–479 (2017).
Knudsen, K., Krogh, K., Ostergaard, K. & Borghammer, P. Constipation in Parkinson’s disease: subjective symptoms, objective markers, and new perspectives. Mov. Disord. 32, 94–105 (2017).
Lewitan, A., Nathanson, L. & Slade, W. R. Jr. Megacolon and dilatation of the small bowel in parkinsonism. Gastroenterology 17, 367–374 (1951).
Berenyi, M. R. & Schwarz, G. S. Megasigmoid syndrome in diabetes and neurologic disease. Review of 13 cases. Am. J. Gastroenterol. 47, 311–320 (1967).
Caplan, L. H., Jacobson, H. G., Rubinstein, B. M. & Rotman, M. Z. Megacolon and volvulus in Parkinson’s disease. Radiology 85, 73–79 (1965).
Rosenthal, M. J. & Marshall, C. E. Sigmoid volvulus in association with parkinsonism. Report of four cases. J. Am. Geriatr. Soc. 35, 683–684 (1987).
Giudicessi, J. R., Ackerman, M. J. & Camilleri, M. Cardiovascular safety of prokinetic agents: a focus on drug-induced arrhythmias. Neurogastroenterol. Motil. 30, e13302 (2018).
Stocchi, F. et al. Anorectal function in multiple system atrophy and Parkinson’s disease. Mov. Disord. 15, 71–76 (2000).
Mukhtar, S., Imran, R., Zaheer, M. & Tariq, H. Frequency of non-motor symptoms in Parkinson’s disease presenting to tertiary care centre in Pakistan: an observational, cross-sectional study. BMJ Open 8, e019172 (2018).
Kim, J. S., Sung, H. Y., Lee, K. S., Kim, Y. I. & Kim, H. T. Anorectal dysfunctions in Parkinson’s disease. J. Neurol. Sci. 310, 144–151 (2011).
Lubomski, M., Davis, R. L. & Sue, C. M. The gut microbiota: a novel therapeutic target in Parkinson’s disease? Parkinsonism Relat. Disord. 66, 265–266 (2019).
Lubomski, M. et al. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. https://doi.org/10.1007/s00415-019-09320-1 (2019).
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J. & Balskus, E. P. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364, eaau6323 (2019).
Perez, M. et al. Tyramine biosynthesis is transcriptionally induced at low pH and improves the fitness of Enterococcus faecalis in acidic environments. Appl. Microbiol. Biotechnol. 99, 3547–3558 (2015).
Dutta, S. K. et al. Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J. Neurogastroenterol. Motil. 25, 363–376 (2019).
Sampson, T. R. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480 (2016).
Cirstea, M. S. et al. Microbiota composition and metabolism are associated with gut function in Parkinson’s disease. Mov. Disord. https://doi.org/10.1002/mds.28052 (2020).
Keshavarzian, A., Engen, P., Bonvegna, S. & Cilia, R. The gut microbiome in Parkinson’s disease: a culprit or a bystander? Prog. Brain Res. 252, 357–450 (2020).
Weis, S. et al. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis. 5, 28 (2019).
Hill-Burns, E. M. et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 32, 739–749 (2017).
Robertson, D. R. et al. The influence of levodopa on gastric emptying in healthy elderly volunteers. Eur. J. Clin. Pharmacol. 42, 409–412 (1992).
Waller, D. G., Roseveare, C., Renwick, A. G., Macklin, B. & George, C. F. Gastric emptying in healthy volunteers after multiple doses of levodopa. Br. J. Clin. Pharmacol. 32, 691–695 (1991).
Marrinan, S. L. et al. A randomized, double-blind, placebo-controlled trial of camicinal in Parkinson’s disease. Mov. Disord. 33, 329–332 (2018).
Muller, T. et al. Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin. Neuropharmacol. 29, 61–67 (2006).
Nyholm, D. & Lennernas, H. Irregular gastrointestinal drug absorption in Parkinson’s disease. Expert. Opin. Drug Metab. Toxicol. 4, 193–203 (2008).
Perni, M. et al. Multistep inhibition of alpha-synuclein aggregation and toxicity in vitro and in vivo by trodusquemine. ACS Chem. Biol. 13, 2308–2319 (2018).
Perni, M. et al. A natural product inhibits the initiation of alpha-synuclein aggregation and suppresses its toxicity. Proc. Natl Acad. Sci. USA 114, E1009–E1017 (2017).
Hauser, R. A. et al. Targeting neurons in the gastrointestinal tract to treat Parkinson’s disease. Clin. Parkinsonism Relat. Disord. 1, 2–7 (2019).
West, C. L. et al. Colonic motility and jejunal vagal afferent firing rates are decreased in aged adult male mice and can be restored by an aminosterol. Front. Neurosci. 13, 955 (2019).
Lee, C. R. & Tepper, J. M. Basal ganglia control of substantia nigra dopaminergic neurons. J. Neural Transm. Suppl. 73, 71–90 (2009).
Bove, C. & Travagli, R. A. Neurophysiology of the brain stem in Parkinson’s disease. J. Neurophysiol. 121, 1856–1864 (2019).
Rommelfanger, K. S. & Wichmann, T. Extrastriatal dopaminergic circuits of the basal ganglia. Front. Neuroanat. 4, 139 (2010).
Dickson, D. W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 46 (Suppl. 1), 30–33 (2018).
McGregor, M. M. & Nelson, A. B. Circuit mechanisms of Parkinson’s disease. Neuron 101, 1042–1056 (2019).
Galvan, A., Devergnas, A. & Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 9, 5 (2015).
Fereshtehnejad, S. M., Zeighami, Y., Dagher, A. & Postuma, R. B. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140, 1959–1976 (2017).
Browning, K. N., Renehan, W. E. & Travagli, R. A. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. J. Physiol. 517, 521–532 (1999).
Browning, K. N., Coleman, F. H. & Travagli, R. A. Characterization of pancreas-projecting rat dorsal motor nucleus of vagus neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G950–G955 (2005).
Gao, H. et al. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 1291, 40–52 (2009).
Blake, C. B. & Smith, B. N. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R711–R720 (2014).
Travagli, R. A., Gillis, R. A., Rossiter, C. D. & Vicini, S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am. J. Physiol. 260, G531–G536 (1991).
Marks, J. D., Donnelly, D. F. & Haddad, G. G. Adenosine-induced inhibition of vagal motoneuron excitability: receptor subtype and mechanisms. Am. J. Physiol. 264, L124–L132 (1993).
Travagli, R. A. & Gillis, R. A. Hyperpolarization-activated currents IH and IKIR in rat dorsal motor nucleus of the vagus neurons in vitro. J. Neurophysiol. 71, 1308–1317 (1994).
Smith, B. N., Dou, P., Barber, W. D. & Dudek, F. E. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J. Physiol. 512, 149–162 (1998).
Goldberg, J. A. et al. Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson’s disease. Nat. Neurosci. 15, 1414–1421 (2012).
Ballanyi, K., Doutheil, J. & Brockhaus, J. Membrane potential and microenvironment of rat dorsal vagal cells in vitro during energy depletion. J. Physiol. 495, 769–784 (1996).
Kulik, A., Trapp, S. & Ballanyi, K. Ischemia but not anoxia evokes vascicular and Ca2+-independent glutamate release in the dorsal vagal complex in vitro. J. Neurophysiol. 83, 2905–2915 (2000).
Trapp, S., Luekermann, M., Brooks, P. A. & Ballanyi, K. Acidosis of rat dorsal vagal neurons in situ during spontaneous and evoked activity. J. Physiol. 496, 695–710 (1996).
Dean, J. B., Gallman, E. A. & Millhorn, D. E. Electrophysiology and morphology of CO2-sensitive neurons in dorsal vagal complex studied in vitro. Soc. Neurosci. 507, 511 (1993).
Dean, J. B. & Mulkey, D. K. Continuous intracellular recording from mammalian neurons exposed to hyperbaric helium, oxygen or air. J. Appl. Physiol. 89, 807–822 (2000).
Lasser-Katz, E. et al. Mutant alpha-synuclein overexpression induces stressless pacemaking in vagal motoneurons at risk in Parkinson’s disease. J. Neurosci. 37, 47–57 (2017).
Bauer, S., Hay, M., Amilhon, B., Jean, A. & Moyse, E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience 130, 75–90 (2005).
Charrier, C. et al. Characterization of neural stem cells in the dorsal vagal complex of adult rat by in vivo proliferation labeling and in vitro neurosphere assay. Neuroscience 138, 5–16 (2006).
Gritti, A. et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 16, 1091–1100 (1996).
Subramaniam, M. et al. Mutant alpha-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J. Neurosci. 34, 13586–13599 (2014).
Zheng, Z. & Travagli, R. A. Dopamine effects on identified rat vagal motoneurons. Am. J. Physiol. Gastrointest. Liver Physiol 292, G1002–G1008 (2007).
Wang, X., Pinol, R. A., Byrne, P. & Mendelowitz, D. Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J. Neurosci. 34, 6182–6189 (2014).
Coon, E. A., Cutsforth-Gregory, J. K. & Benarroch, E. E. Neuropathology of autonomic dysfunction in synucleinopathies. Mov. Disord. https://doi.org/10.1002/mds.27186 (2018).
Benarroch, E. E. The clinical approach to autonomic failure in neurological disorders. Nat. Rev. Neurol. 10, 396–407 (2014).
Postuma, R. B. & Berg, D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 12, 622–634 (2016).
Goldstein, D. S. Dysautonomia in Parkinson disease. Compr. Physiol. 4, 805–826 (2014).
Grinberg, L. T., Rueb, U., Alho, A. T. & Heinsen, H. Brainstem pathology and non-motor symptoms in PD. J. Neurol. Sci. 289, 81–88 (2010).
Acknowledgements
The authors thank the NIH (grants DK 55530 and DK 124098 to R.A.T., DK 111667 to K.N.B. and DK 115950 and 122280 to M.C.), the National Parkinson Foundation (R.A.T.), and the Michael J. Fox Foundation for Parkinson’s Disease (R.A.T.) for their support. The authors also thank C. M. Travagli, Z. Travagli and W. N. Browning for support and encouragement.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.C. serves as a member of Scientific Advisory Board of Enterin, a company that is developing a medication for Parkinson Disease. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks P. Derkinderen, M. Gershon and R. Liddle for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Travagli, R.A., Browning, K.N. & Camilleri, M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 17, 673–685 (2020). https://doi.org/10.1038/s41575-020-0339-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-0339-z
- Springer Nature Limited
This article is cited by
-
Helicobacter pylori infection and Parkinson’s Disease: etiology, pathogenesis and levodopa bioavailability
Immunity & Ageing (2024)
-
Effects of dietary intervention on human diseases: molecular mechanisms and therapeutic potential
Signal Transduction and Targeted Therapy (2024)
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Intensive training programme improves handwriting in a community cohort of people with Parkinson’s disease
Irish Journal of Medical Science (1971 -) (2024)
-
Neuroimmune Connectomes in the Gut and Their Implications in Parkinson’s Disease
Molecular Neurobiology (2024)