Abstract
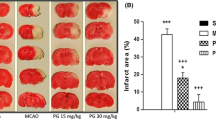
The present study has been aimed to explore the different secondary messengers of the inflammatory pathway NF-κB, kinases (JNK, P38MAPK, GSK3β/βcatenin), apoptosis pathway (Caspase-3 and AIF), and neuronal survival pathway (BDNF) in order to understand the neuroprotective mechanism of aqueous extract of Tribulus terrestris (AQTT). In primary cortical neurons, the ischemic condition was induced through oxygen–glucose deprivation (OGD). Anti-inflammatory activity of AQTT was evaluated in formalin induced inflammation model and carrageenan-induced paw edema test. The bilateral common carotid artery occlusion model was employed for whole animal studies. Treatment of AQTT (100 mg/kg) significantly reduced the inflammation induced by formalin and carrageenan. The neuroprotective mechanism of AQTT (50 and 100 mg/kg) was assessed by pre- and post-administration. The results indicate down regulation of kinases and NFkB, suggesting possible anti-inflammatory activity of AQTT. Additionally, AQTT down regulated both caspase dependent and independent apoptotic pathways suggesting its possible anti-apoptotic activity. The treatment of AQTT also reduced GSK3β levels and increased p-Ser9 GSK3β levels; stabilizing the unphosphorylated form of β-catenin and its translocation into the nucleus suggesting role of AQTT in neuronal survival and GSK3β mediated anti-inflammatory property. In comparison to pretreatment, post treatment of AQTT had lesser effects indicating tribulusterine standardized AQTT may have prophylactic effect. This study can be concluded with the thesis that AQTT has neuroprotective effect through alternating neuroinflammation, apoptosis, and promoting neuron survival. Being that it produced better effect with pretreatment, exploring this with thrombolytic drugs will be beneficial. For the first time AQTT has been reported for this indication.







Similar content being viewed by others
References
Chhatre S, Nesari T, Kanchan D et al (2014) Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev 8:45. https://doi.org/10.4103/0973-7847.125530
Ranjithkumar R, Prathab Balaji S, Balaji B et al (2013) Standardized aqueous Tribulus terristris (Nerunjil) extract attenuates hyperalgesia in experimentally induced diabetic neuropathic pain model: role of oxidative stress and inflammatory mediators. Phyther Res 27:1646–1657. https://doi.org/10.1002/ptr.4915
Reshma PL, Sainu NS, Mathew AK, Raghu KG (2016) Mitochondrial dysfunction in H9c2 cells during ischemia and amelioration with Tribulus terrestris L. Life Sci 152:220–230. https://doi.org/10.1016/j.lfs.2016.03.055
Ehrman TM, Barlow DJ, Hylands PJ (2010) In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorg Med Chem 18:2204–2218. https://doi.org/10.1016/j.bmc.2010.01.070
Ko H-J, Ahn E-K, Oh JS (2015) N-trans-ρ-caffeoyl tyramine isolated from Tribulus terrestris exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 cells. Int J Mol Med 36:1042–1048. https://doi.org/10.3892/ijmm.2015.2301
Zhang S, Li H, Yang S-J (2011) Tribulosin suppresses apoptosis via PKC epsilon and ERK1/2 signaling pathway during hypoxia/reoxygenation in neonatal rat ventricular cardiac myocytes. J Asian Nat Prod Res 13:1135–1145. https://doi.org/10.1080/10286020.2011.627327
Jiang E, Li H, Chen J, Yang S (2011) Protection by the gross saponins of Tribulus terrestris against cerebral ischemic injury in rats involves the NF-κB pathway. Acta Pharm Sin B 1:21–26. https://doi.org/10.1016/j.apsb.2011.04.009
Gautam M, Ramanathan M (2018) Saponins of Tribulus terrestris attenuated neuropathic pain induced with vincristine through central and peripheral mechanism. Inflammopharmacology. https://doi.org/10.1007/s10787-018-0502-0
George PM, Steinberg GK (2015) Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87:297–309. https://doi.org/10.1016/j.neuron.2015.05.041
Denes A, Thornton P, Rothwell NJ, Allan SM (2010) Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun 24:708–723. https://doi.org/10.1016/j.bbi.2009.09.010
Rodrigues SF, Granger DN (2014) Leukocyte-mediated tissue injury in ischemic stroke. Curr Med Chem 21:2130–2137
Irving EA, Barone FC, Reith AD et al (2000) Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res 77:65–75
Morrison DK, Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19:91–118. https://doi.org/10.1146/annurev.cellbio.19.111401.091942
Mirabelli-Badenier M, Braunersreuther V, Lenglet S et al (2012) Pathophysiological role of inflammatory molecules in paediatric ischaemic brain injury. Eur J Clin Invest 42:784–794. https://doi.org/10.1111/j.1365-2362.2012.02640.x
Dutta J, Fan Y, Gupta N et al (2006) Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 25:6800–6816. https://doi.org/10.1038/sj.onc.1209938
Beurel E, Jope RS (2009) Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflamm. https://doi.org/10.1186/1742-2094-6-9
Cheng Y-L, Wang C-Y, Huang W-C et al (2009) Staphylococcus aureus induces microglial inflammation via a glycogen synthase kinase 3beta-regulated pathway. Infect Immun. https://doi.org/10.1128/IAI.00176-09
Zhang Q-G, Wang R, Khan M et al (2008) Role of Dickkopf-1, an antagonist of the Wnt/-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of Tau phosphorylation. J Neurosci 28:8430–8441. https://doi.org/10.1523/JNEUROSCI.2752-08.2008
Wisniewska MB (2013) Physiological role of β-catenin/TCF signaling in neurons of the adult brain. Neurochem Res 38:1144–1155. https://doi.org/10.1007/s11064-013-0980-9
Wang M-J, Huang H-Y, Chen W-F et al (2010) Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J Neuroinflamm. https://doi.org/10.1186/1742-2094-7-99
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. https://doi.org/10.1146/annurev.cellbio.20.010403.113126
Shimizu H, Julius MA, Giarré M et al (1997) Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ 8:1349–1358
Broughton BRS, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339. https://doi.org/10.1161/STROKEAHA.108.531632
Ferrer I (2006) Apoptosis: future targets for neuroprotective strategies. Cerebrovasc Dis 21:9–20. https://doi.org/10.1159/000091699
Waterhouse EG, An JJ, Orefice LL et al (2012) BDNF promotes differentiation and maturation of adult-born neurons through gabaergic transmission. J Neurosci 32:14318–14330. https://doi.org/10.1523/JNEUROSCI.0709-12.2012
Gessner WP, Brossi A, Bembenek ME, Abell CW (1988) β-Carbolines from Japanese sake and soy sauce: synthesis and biological activity of flazin and yellow substance YS (perlolyrine). Arch Pharm (Weinheim) 321:95–98
Nada SE, Shah ZA (2012) Preconditioning with Ginkgo biloba (EGb 761®) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis 46:180–189. https://doi.org/10.1016/j.nbd.2012.01.006
Madineni A, Alhadidi Q, Shah ZA (2016) Cofilin inhibition restores neuronal cell death in oxygen–glucose deprivation model of ischemia. Mol Neurobiol 53:867–878. https://doi.org/10.1007/s12035-014-9056-3
Olechnowicz SWZ, Fedele AO, Peet DJ (2012) Hypoxic induction of the regulator of G-protein signalling 4 gene is mediated by the hypoxia-inducible factor pathway. PLoS ONE 7:e44564. https://doi.org/10.1371/journal.pone.0044564
Saravanan PB, Shanmuganathan MV, Ramanathan M (2015) Telmisartan attenuated LPS-induced neuroinflammation in human IMR-32 neuronal cell line via SARM in AT1R independent mechanism. Life Sci 130:88–96. https://doi.org/10.1016/j.lfs.2015.03.005
Tulsulkar J, Shah ZA (2013) Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int 62:189–197. https://doi.org/10.1016/j.neuint.2012.11.017
Ranjithkumar R, Premnath P, Ramanathan M (2015) Measurement of inflammatory mediators at different time intervals after neuronal injury induced by bilateral common carotid artery occlusion model. J Pharm Sci Res 7(9):662
Nada SE, Tulsulkar J, Shah ZA (2014) Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761®) after permanent ischemic stroke in mice. Mol Neurobiol 49:945–956. https://doi.org/10.1007/s12035-013-8572-x
Cai M, Phan P-TT, Hong JG et al (2012) The neuroprotective effect of eupatilin against ischemia/reperfusion-induced delayed neuronal damage in mice. Eur J Pharmacol 689:104–110. https://doi.org/10.1016/j.ejphar.2012.05.042
Javadov S, Jang S, Agostini B (2014) Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol Ther 144:202–225. https://doi.org/10.1016/j.pharmthera.2014.05.013
Jiang M, Li J, Peng Q et al (2014) Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflamm 11:167. https://doi.org/10.1186/s12974-014-0167-6
Kang SY, Jung HW, Nam JH et al (2017) Effects of the fruit extract of Tribulus terrestris on skin inflammation in mice with oxazolone-induced atopic dermatitis through regulation of calcium channels, Orai-1 and TRPV3, and mast cell activation. Evid Based Complement Altern Med 2017:8312946. https://doi.org/10.1155/2017/8312946
Jiang Y-H, Guo J-H, Wu S, Yang C-H (2017) Vascular protective effects of aqueous extracts of Tribulus terrestris on hypertensive endothelial injury. Chin J Nat Med 15:606–614. https://doi.org/10.1016/S1875-5364(17)30088-2
Lee HH, Ahn E-K, Hong S-S, Oh JS (2017) Anti-inflammatory effect of tribulusamide D isolated from Tribulus terrestris in lipopolysaccharide-stimulated RAW264.7 macrophages. Mol Med Rep 16:4421–4428. https://doi.org/10.3892/mmr.2017.7208
Oh J, Baik S, Ahn E et al (2012) Anti-inflammatory activity of Tribulus terrestris in RAW264.7 Cells (54.2). J Immunol 188(1 supplement):54.2
Jiang Y-H, Jiang L-Y, Wu S et al (2018) Proteomic analysis reveals the renoprotective effect of Tribulus terrestris against obesity-related glomerulopathy in rats. Biol Pharm Bull 41:1430–1439. https://doi.org/10.1248/bpb.b18-00304
Ozawa H, Shioda S, Dohi K et al (1999) Delayed neuronal cell death in the rat hippocampus is mediated by the mitogen-activated protein kinase signal transduction pathway. Neurosci Lett 262:57–60
Gao Y, Signore AP, Yin W et al (2005) Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab 25:694–712. https://doi.org/10.1038/sj.jcbfm.9600062
Jiang Y, Yang C, Li W et al (2016) Aqueous extracts of Tribulus terrestris protects against oxidized low-density lipoprotein-induced endothelial dysfunction. Chin J Integr Med 22:193–200. https://doi.org/10.1007/s11655-015-2321-0
Borran M, Minaiyan M, Zolfaghari B, Mahzouni P (2017) Protective effect of Tribulus terrestris fruit extract on cerulein-induced acute pancreatitis in mice. Avicenna J Phytomed 7:250–260
Darshit BS, Ramanathan M (2017) Glycogen synthase kinase-3: a potential target for drug discovery in the treatment of neurodegenerative disorders. Curr Enzym Inhib 13:107–128
Jope RS, Cheng Y, Lowell JA et al (2017) Stressed and inflamed, can GSK3 be blamed? Trends Biochem Sci 42(3):180–192
Beurel E, Michalek SM, Jope RS (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31(1):24–31
Jacobs KM, Bhave SR, Ferraro DJ et al (2012) GSK-3: a bifunctional role in cell death pathways. Int J Cell Biol 2012:1–11. https://doi.org/10.1155/2012/930710
Chen BH, Ahn JH, Park JH et al (2017) Transient cerebral ischemia alters GSK-3β and p-GSK-3β immunoreactivity in pyramidal neurons and induces p-GSK-3β expression in astrocytes in the gerbil hippocampal CA1 area. Neurochem Res 42:2305–2313. https://doi.org/10.1007/s11064-017-2245-5
Darshit BS, Ramanathan M (2016) Activation of AKT1/GSK-3β/β-catenin-TRIM11/survivin pathway by novel GSK-3β inhibitor promotes neuron cell survival: study in differentiated SH-SY5Y cells in OGD model. Mol Neurobiol 53:6716–6729. https://doi.org/10.1007/s12035-015-9598-z
Libro R, Bramanti P, Mazzon E (2016) The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci 158:78–88. https://doi.org/10.1016/j.lfs.2016.06.024
Jordan J, de Groot PWJ, Galindo MF (2011) Mitochondria: the headquarters in ischemia-induced neuronal death. Cent Nerv Syst Agents Med Chem 11:98–106
Thal SE, Zhu C, Thal SC et al (2011) Role of apoptosis inducing factor (AIF) for hippocampal neuronal cell death following global cerebral ischemia in mice. Neurosci Lett 499:1–3. https://doi.org/10.1016/j.neulet.2011.05.016
Acknowledgements
This study was financially supported by University of Toledo, OHIO, USA, PSG Sons & Charities, and PSG College of Pharmacy, Coimbatore, India. We are grateful to Dr. Sivaram Hariharan for his helpful grammar corrections on the manuscript. We thank N. Rama Varier of Ayurvedic foundation for providing gift sample of T.terrestris aqueous extract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All protocols were approved by the Institutional Animal Ethical Committee, PSG IMS&R, and experiments were performed in accordance to the CPCSEA guidelines for ethical use of animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ranjithkumar, R., Alhadidi, Q., Shah, Z.A. et al. Tribulusterine Containing Tribulus terrestris Extract Exhibited Neuroprotection Through Attenuating Stress Kinases Mediated Inflammatory Mechanism: In Vitro and In Vivo Studies. Neurochem Res 44, 1228–1242 (2019). https://doi.org/10.1007/s11064-019-02768-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02768-7