Abstract
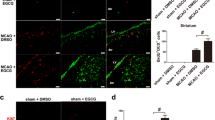
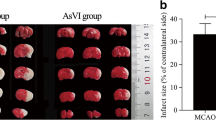
Stroke is the fourth leading cause of death and a major cause of disability in stroke survivors. Studies have underlined the importance of repair mechanisms in the recovery phase of stroke. Neurogenesis in response to brain injury is one of the regeneration processes that, if enhanced, may offer better stroke treatment alternatives. Previously, we have demonstrated antioxidant, neuritogenic, and angiogenic properties of Ginkgo biloba/EGb 761® (EGb 761) in different mouse models of stroke. In the present study, we were interested to study whether EGb 761 could protect mice from permanent middle cerebral artery occlusion (pMCAO) and enhance neurogenesis. EGb 761 pre- and posttreated mice had lower infarct volume and improved motor skills with enhanced proliferation of neuronal stem/progenitor cells (NSPCs) at 24 h and 7 days posttreatment. Netrin-1 and its receptors (DCC and UNC5B) that mediate axonal attraction and repulsion were observed to be overexpressed in NSPCs only, implying that netrin-1 and its receptors might have partly played a role in enhanced neurogenesis. Interestingly, in heme oxygenase 1 knockout mice (HO1−/−), neurogenesis was significantly lower than in vehicle-treated mice at day 8. Furthermore, EGb 761 posttreated mice also demonstrated heme oxygenase 1 (HO1)-activated pathway of phosphorylated glycogen synthase kinase 3 α/β (p-GSK-3 α/β), collapsin response mediator protein 2 (CRMP-2), semaphorin3A (SEMA3A), and Wnt, suggesting probable signaling pathways involved in proliferation, differentiation, and migration of NSPCs. Together, these results propose that EGb 761 not only has antioxidant, neuritogenic, and angiogenic properties, but can also augment the repair and regeneration mechanisms following stroke.






Similar content being viewed by others
Abbreviations
- BrdU:
-
5-Bromo-2′-deoxyuridine
- CRMP-2:
-
Collapsin response mediator protein 2
- EGb 761:
-
Ginkgo biloba/EGb 761®
- HO1:
-
Heme oxygenase 1
- PMCAO:
-
Permanent middle cerebral artery occlusion
- TTC:
-
Triphenyltetrazolium chloride
- NSPCs:
-
Neuronal stem/progenitor cells
- SEMA3A:
-
Semaphorin3A
- DCC:
-
Deleted in colorectal cancer
- UNC5B:
-
Uncoordinated gene 5B
- BDNF:
-
Brain-derived neurotrophic factor
- GSK-3 α/β:
-
Glycogen synthase kinase 3 α and β
- p-GSK-3 α/β:
-
Phosphorylated form of glycogen synthase kinase-3 α/β
References
Greenberg DA (2007) Neurogenesis and stroke. CNS & neurological disorders drug targets 6(5):321–325
Niv F, Keiner S, Krishna-K, Witte OW, Lie DC, Redecker C (2012) Aberrant neurogenesis after stroke a retroviral cell labeling study. Stroke; a journal of cerebral circulation 43 (9):2468-+. doi: 10.1161/Strokeaha.112.660977
Guo H, Zhao Z, Yang Q, Wang M, Bell RD, Wang S, Chow N, Davis TP, Griffin JH, Goldman SA, Zlokovic BV (2013) An activated protein c analog stimulates neuronal production by human neural progenitor cells via a PAR1–PAR3–S1PR1–Akt pathway. J Neurosci 33(14):6181–6190. doi:10.1523/JNEUROSCI.4491-12.2013
Jobe EM, McQuate AL, Zhao X (2012) Crosstalk among epigenetic pathways regulates neurogenesis. Front Neurosci 6:59. doi:10.3389/fnins.2012.00059
Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A (2006) Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. The J Comp Neurol 494(3):415–434. doi:10.1002/cne.20798
Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24(3):739–747. doi:10.1634/stemcells.2005-0281
Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M (2001) Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke; a journal of cerebral circulation 32(8):1890–1896
Kernie SG, Parent JM (2010) Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis 37(2):267–274. doi:10.1016/j.nbd.2009.11.002
Shruster A, Ben-Zur T, Melamed E, Offen D (2012) Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PloS one 7 (7). doi:10.1371/journal.pone.0040843
Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF (2013) Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurological function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther 4(3):73. doi:10.1186/scrt224
Putman DM, Liu KY, Broughton HC, Bell GI, Hess DA (2012) Umbilical cord blood-derived aldehyde dehydrogenase-expressing progenitor cells promote recovery from acute ischemic injury. Stem Cells 30(10):2248–2260. doi:10.1002/stem.1206
Shen WC, Liang CJ, Wu VC, Wang SH, Young GH, Lai IR, Chien CL, Wang SM, Wu KD, Chen YL (2013) Endothelial progenitor cells derived from Wharton's jelly of the umbilical cord reduces ischemia-induced hind limb injury in diabetic mice by inducing HIF-1alpha/IL-8 expression. Stem Cells Dev 22(9):1408–1418. doi:10.1089/scd.2012.0445
Shah ZA, Nada SE, Dore S (2011) Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 180:248–255. doi:10.1016/j.neuroscience.2011.02.031
Nada SE, Shah ZA (2012) Preconditioning with Ginkgo biloba (EGb 761(R)) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis 46(1):180–189. doi:10.1016/j.nbd.2012.01.006
Chen S, Sega M, Agarwal A (2004) “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. BioTechniques 37(1):84–86, 88–89
Nada SE, Tulsulkar J, Raghavan A, Hensley K, Shah ZA (2012) A derivative of the CRMP2 binding compound lanthionine ketimine provides neuroprotection in a mouse model of cerebral ischemia. Neurochem Int 61(8):1357–1363. doi:10.1016/j.neuint.2012.09.013
Waterhouse EG, An JJ, Orefice LL, Baydyuk M, Liao GY, Zheng K, Lu B, Xu BJ (2012) BDNF Promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci 32(41):14318–14330. doi:10.1523/Jneurosci.0709-12.2012
Font MA, Arboix A, Krupinski J (2010) Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev 6(3):238–244. doi:10.2174/157340310791658802
Xiong Y, Mahmood A, Chopp M (2010) Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 11(3):298–308
Xiong Y, Mahmood A, Chopp M (2010) Neurorestorative treatments for traumatic brain injury. Discovery medicine 10(54):434–442
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. doi:10.1016/j.neuron.2011.05.001
de Castro F (2003) Chemotropic molecules: guides for axonal pathfinding and cell migration during CNS development. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the. Am Physiol Soc 18:130–136
Sun L, Lee J, Fine HA (2004) Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest 113(9):1364–1374. doi:10.1172/JCI20001
Manitt C, Thompson KM, Kennedy TE (2004) Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res 77(5):690–700. doi:10.1002/jnr.20199
Bradford D, Cole SJ, Cooper HM (2009) Netrin-1: diversity in development. Int J Biochem Cell Biol 41(3):487–493. doi:10.1016/j.biocel.2008.03.014
Lange C, Mix E, Frahm J, Glass A, Muller J, Schmitt O, Schmole AC, Klemm K, Ortinau S, Hubner R, Frech MJ, Wree A, Rolfs A (2011) Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells. Neurosci Lett 488(1):36–40. doi:10.1016/j.neulet.2010.10.076
Dong S, Zeng Q, Mitchell ES, Xiu J, Duan Y, Li C, Tiwari JK, Hu Y, Cao X, Zhao Z (2012) Curcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticity. PLoS One 7(2):e31211. doi:10.1371/journal.pone.0031211
Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD (2012) Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell stem cell 11(1):23–35. doi:10.1016/j.stem.2012.03.016
Arakawa H (2004) Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer 4(12):978–987. doi:10.1038/nrc1504
Furne C, Rama N, Corset V, Chedotal A, Mehlen P (2008) Netrin-1 is a survival factor during commissural neuron navigation. Proc Natl Acad Sci U S A 105(38):14465–14470. doi:10.1073/pnas.0803645105
Tang X, Jang SW, Okada M, Chan CB, Feng Y, Liu Y, Luo SW, Hong Y, Rama N, Xiong WC, Mehlen P, Ye K (2008) Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol 10(6):698–706. doi:10.1038/ncb1732
Masuda T, Watanabe K, Sakuma C, Ikenaka K, Ono K, Yaginuma H (2008) Netrin-1 acts as a repulsive guidance cue for sensory axonal projections toward the spinal cord. J Neurosci 28(41):10380–10385. doi:10.1523/JNEUROSCI.1926-08.2008
Lu H, Wang Y, He X, Yuan F, Lin X, Xie B, Tang G, Huang J, Tang Y, Jin K, Chen S, Yang GY (2012) Netrin-1 hyperexpression in mouse brain promotes angiogenesis and long-term neurological recovery after transient focal ischemia. Stroke 43(3):838–843. doi:10.1161/STROKEAHA.111.635235
Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH (2008) Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci 28(5):1099–1108. doi:10.1523/JNEUROSCI.4906-07.2008
Tsuchiya A, Hayashi T, Deguchi K, Sehara Y, Yamashita T, Zhang H, Lukic V, Nagai M, Kamiya T, Abe K (2007) Expression of netrin-1 and its receptors DCC and neogenin in rat brain after ischemia. Brain Res 1159:1–7. doi:10.1016/j.brainres.2006.12.096
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ (2013) Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res 91(1):30–41. doi:10.1002/jnr.23138
Louhivuori V, Vicario A, Uutela M, Rantamaki T, Louhivuori LM, Castren E, Tongiorgi E, Akerman KE, Castren ML (2011) BDNF and TrkB in neuronal differentiation of Fmr1-knockout mouse. Neurobiol Dis 41(2):469–480. doi:10.1016/j.nbd.2010.10.018
Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A (2013) Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One 8(1):e55039. doi:10.1371/journal.pone.0055039
Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG (2007) Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 38(7):2165–2172. doi:10.1161/STROKEAHA.106.477331
Suzuki Y, Nakagomi S, Namikawa K, Kiryu-Seo S, Inagaki N, Kaibuchi K, Aizawa H, Kikuchi K, Kiyama H (2003) Collapsin response mediator protein-2 accelerates axon regeneration of nerve-injured motor neurons of rat. J Neurochem 86(4):1042–1050
Schmidt EF, Strittmatter SM (2007) The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol 600:1–11. doi:10.1007/978-0-387-70956-7_1
Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y (2005) Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer's disease. Genes Cells 10(2):165–179. doi:10.1111/j.1365-2443.2005.00827.x
McLoon LK (2009) A new role for satellite cells: control of reinnervation after muscle injury by semaphorin 3A. Focus on “possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation”. Am J Physiol Cell Physiol 297(2):C227–C230. doi:10.1152/ajpcell.00256.2009
Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, Molday RS, Goshima Y, Hong K (2008) Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron 58(5):694–707. doi:10.1016/j.neuron.2008.03.017
Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM (2000) Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol 149(2):411–422
Castellani V, De Angelis E, Kenwrick S, Rougon G (2002) Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J 21(23):6348–6357
Marin O, Rubenstein JL (2001) A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2(11):780–790. doi:10.1038/35097509
Castellani V, Rougon G (2002) Control of semaphorin signaling. Curr Opin Neurobiol 12(5):532–541
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837. doi:10.1093/eurheartj/ehr304, 837a-837d
Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG, Nisoli E (2011) Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem 116(6):1148–1159. doi:10.1111/j.1471-4159.2011.07171.x
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11(8):539–551. doi:10.1038/nrn2870
Zheng H, Ying H, Wiedemeyer R, Yan H, Quayle SN, Ivanova EV, Paik JH, Zhang H, Xiao Y, Perry SR, Hu J, Vinjamoori A, Gan B, Sahin E, Chheda MG, Brennan C, Wang YA, Hahn WC, Chin L, DePinho RA (2010) PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer cell 17(5):497–509. doi:10.1016/j.ccr.2010.03.020
Valvezan AJ, Klein PS (2012) GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Front Mol Neurosci 5:1. doi:10.3389/fnmol.2012.00001
Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12(9):1097–1105. doi:10.1038/nn.2360
Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD Jr, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, Abraham NG (2013) Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4(2):28. doi:10.1186/scrt176
Tulsulkar J, Shah ZA (2012) Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int 62(2):189–197. doi:10.1016/j.neuint.2012.11.017
Christodoulides N, Durante W, Kroll MH, Schafer AI (1995) Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation 91(9):2306–2309
Reierson GW, Guo S, Mastronardi C, Licinio J, Wong ML (2011) cGMP signaling, phosphodiesterases and major depressive disorder. Curr Neuropharmacol 9(4):715–727. doi:10.2174/157015911798376271
Acknowledgments
This work was supported by a grant from the National Institutes of Health—National Center for Complementary and Alternative Medicine (R00AT004197) and start-up funds from the University of Toledo to ZAS. The authors would like to thank Charisse N. Montgomery for her assistance in the manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shadia E. Nada and Jatin Tulsulkar contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nada, S.E., Tulsulkar, J. & Shah, Z.A. Heme Oxygenase 1-Mediated Neurogenesis Is Enhanced by Ginkgo biloba (EGb 761®) After Permanent Ischemic Stroke in Mice. Mol Neurobiol 49, 945–956 (2014). https://doi.org/10.1007/s12035-013-8572-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8572-x