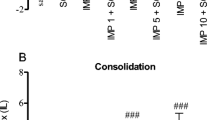
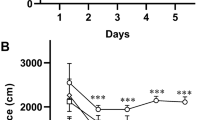
One of the hallmarks of Alzheimer’s disease (AD) is extracellular deposition of amyloid-β peptides, particularly in the hippocampus. Despite the antioxidant properties of taurine, its neuroprotective potential against amyloid-β accumulation in scopolamine-induced AD model remain unclear. In such a model, we aimed to assess the effects of taurine on passive avoidance memory impairment, accumulation of congophilic amyloid-β plaques, and neuronal density in the rat hippocampus. Rats, except the control group, were i.p. injected with 3 mg/kg scopolamine. The pretreated and treated groups were also injected with taurine (25, 50, or 100 mg/kg/day, i.p.) for 14 days before or after scopolamine introduction. All rats (except the control group) were tested for the passive avoidance reaction 24 h after the last drug injection. For histological analysis, hippocampal sections were stained with Congo red and cresyl violet. Administration of taurine for 14 days, both before and after scopolamine injection, significantly alleviated passive avoidance memory impairment. Pretreatment with taurine in any of the mentioned dosages significantly decreased the number of congophilic amyloid-β plaques in the rat hippocampus, including the CA3 area. Taurine also reduced scopolamine-induced neuronal loss in the hippocampus.
Similar content being viewed by others
References
S. H. Choi, S. Aid, L. Caracciolo, et al., “Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease,” J. Neurochem.,124, 59–68 (2013).
P. Xu, K. Wang, C. Lu, et al., “Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice,” Life Sci.,174, 21–27 (2017).
H. Javed, A. Khan, K. Vaibhav, et al., “Taurine amelio- rates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer’s type (SDAT) caused by intracerebroventricular streptozotocin in rats,” Neurol. Res.,34, 2181–2192 (2013).
X. Li, H. F. Yuan, Q. K. Quan, et al., “Scavenging effect of Naoerkang on amyloid beta-peptide deposition in the hippocampus in a rat model of Alzheimer’s disease,” Chin. J. Integr. Med.,17, 847–853 (2011).
P. Goverdhan, A. Sravanthi, and T. Mamatha, “Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress,” Int. J. Alzheimers Dis.,2012, 974013; doi: https://doi.org/10.1155/2012/974013. (2012).
M. C. Chung, P. Malatesta, P. L. Bosquesi, et al., “Advances in drug design based on the amino acid approach: Taurine analogues for the treatment of CNS diseases,” Pharmaceuticals,5, 1128–1146 (2012).
Q. Sun, H. Hu, W. Wang, et al., “Taurine attenuates amyloid β1-42-induced mitochondrial dysfunction by activating of SIRT1 in SK-N-SH cells,” Biochem. Biophys. Res. Commun.,447, 485–489 (2014).
A. Blokland, A. Sambeth, J. Prickaerts, and W. J.Riedel, “Why an M1 antagonist could be a more selective model for memory impairment than scopolamine,” Front. Neurol.,7, 167 (2016).
D. Y. Choi, Y. J. Lee, S. Y. Lee, et al., “Attenuation of scopolamine-induced cognitive dysfunction by obovatol,” Arch. Pharm. Res.,35, 1279–1286 (2012).
M. Jahanshahi, E. G. Nickmahzar, and F. Babakordi, “The effect of Ginkgo biloba extract on scopolamineinduced apoptosis in the hippocampus of rats,” Anat. Sci. Int.,88, 217–222 (2013).
G. Caletti, D. B. Olguins, E. F. Pedrollo, et al., “Antidepressant effect of taurine in diabetic rats,” Amino Acids,43, 1525–1533 (2012).
S. Seifhosseini, M. Jahanshahi, A. Moghimi, and N. S. Aazami, “The effect of scopolamine on avoidance memory and hippocampal neurons in male wistar rats,” Basic Clin. Neurosci.,3, 9–15 (2011).
S. Mahakizadeh, M. Jahanshahi, K. Haidari, and M. Shahbazi,“Dopamine receptors gene expression in male rat hippocampus after administration of MDMA (Ecstasy),” Int. J. Morphol.,33, 301–308 (2015).
G. Paxinos, and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego (2007).
D. M. Wilcock, M.N. Gordon, and D. Morgan, “Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo Red histochemical stain,” Nat. Protoc.,1, 1591–1595 (2006).
M. Jahanshahi, K. Haidari, S. Mahaki-Zadeh, et al., “Effects of repeated administration of 3,4-methylenedioxymethamphetamine (MDMA) on avoidance memory and cell density in rats’ hippocampus,” Basic Clin. Neurosci.,4, 57–63 (2013).
E. Nikmahzar, M. Jahanshahi, A. Ghaemi, et al., “Hippocampal serotonin-2A receptor-immunoreactive neurons density increases after testosterone therapy in the gonadectomized male mice,” Anat. Cell Biol.,49, 259–272 (2016).
M. Jahanshahi, Y. Sadeghi, and A. Hosseini, “Estimation of astrocyte number in different subfield of rat hippocampus,” Pak. J. Biol. Sci.,9, 1595–1597 (2006).
J. J. Buccafusco,“The revival of scopolamine reversal for the assessment of cognitive-enhancing drugs,” in: Methods of Behavior Analysis in Neuroscience, J. J. Buccafusco (ed.), CRC Press, Boca Raton (2009), pp. 230–329.
A. C. G. Souza, C. A. Bruning, C. I. Acker, et al., “2-Phenylethynyl- butyltellurium enhances learning and memory impaired by scopolamine in mice,” Behav. Pharmacol.,24, 249–254 (2013).
J. M. Gutierres, F. B. Carvalho, M. R. C. Schetinger, et al., “Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamineinduced amnesia in rats,” Int. J. Dev. Neurosci.,33, 88–97 (2014).
J. S. Lee, S. S. Hong, H. G. Kim, et al.,“Gongjin-dan enhances hippocampal memory in a mouse model of scopolamine-induced amnesia,” PLoS One,11, e0159823 (2016).
A. El Idrissi, “Taurine improves learning and retention in aged mice,” Neurosci. Lett.,436, 19–22 (2008).
H. Y. Kim, H. V. Kim, J. H. Yoon, et al., “Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease,” Sci. Rep.,4, 7467 (2014).
S. F. El-Sisi, “The possible protective effect of mefenamic acid, taurine, soy-phytoestrogen extract against scopolamine induced Alzheimer disease in rat,” New York Sci. J.,4, 89–101 (2011).
M. G. Akande, Y. O. Aliu, S. F. Ambali, and J. O. Ayo, “Taurine mitigates cognitive impairment induced by chronic co-exposure of male Wistar rats to chlorpyrifos and lead acetate,” Environ. Toxicol. Pharmacol.,37, 315–325 (2014).
I. A. Adedara, A. O. Abolaji, U. F. Idris, et al., “Neuroprotective influence of taurine on fluorideinduced biochemical and behavioral deficits in rats,” Chem. Biol. Interact.,261, 1–10 (2017).
K. Ito, M. Arko, T. Kawaguchi, et al., “The effect of subacute supplementation of taurine on spatial learning and memory,” Exp. Anim.,58, 175–180 (2009).
K. Ito, M. Arko, T. Kawaguchi, et al., “Intracerebroventricular administration of taurine impairs learning and memory in rats,” Nutr. Neurosci.,15, 70–77 (2012).
K. M. Rodrigue, K. M. Kennedy, M. D. Devous, et al., “β-Amyloid burden in healthy aging regional distribution and cognitive consequences,” Neurology,78, 387–395 (2012).
S. W. Bihaqi, A. P. Singh, and M. Tiwari, “Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and amyloid precursor protein (AβPP) expression in rat brain,” Ind. J. Pharmacol.,44, 593–598 (2012).
I. Santa-María, F. Hernández, F. J. Moreno, and J. Avila, “Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-β-peptide aggregation,” Neurosci. Lett.,429, 91–94 (2007).
P. R. Louzada, L. A. C. Paula, D. L. Mendonca-Silva, et al., “Taurine prevents the neurotoxicity of betaamyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders,” FASEB J.,18, 511–518 (2004).
A. C. Paula-Lima, F. G. De Felice, J. Brito-Moreira, and S. T. Ferreira, “Activation of GABAA receptors by taurine and muscimol blocks the neurotoxicity of β-amyloid in rat hippocampal and cortical neurons,” Neuropharmacology,49, 1140–1148 (2005).
F. Gervais, J. Paquette, C. Morissette, et al., “Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis,” Neurobiol. Aging,28, 537–547 (2007).
P. E. Fraser, J. T. Nguyen, D. T. Chin, and D. A. Kirschner, “Effects of sulfate ions on Alzheimer β/A4 peptide assemblies: implications for amyloid fibrilproteoglycan interactions,” J. Neurochem.,59, 1531–1540 (1992).
R. Hernández-Benítez, H. Pasantes-Morales, I. T. Saldaña, and G. Ramos-Mandujano, “Taurine stimulates proliferation of mice embryonic cultured neural progenitor cells,” J. Neurosci. Res.,88, 1673–1681 (2010).
M. C. Shivaraj, G. Marcy, G. Low, et al., “Taurine induces proliferation of neural stem cells and synapse development in the developing mouse brain,” PLoS One,7, e42935 (2012).
R. Hernández-Benítez, S. D. Vangipuram, G. Ramos-Mandujano, et al., “Taurine enhances the growth of neural precursors derived from fetal human brain and promotes neuronal specification,” Dev. Neurosci.,35, 40–49 (2013).
E. Gebara, F. Udry, S. Sultan, and N. Toni, “Taurine increases hippocampal neurogenesis in aging mice,” Stem Cell Res.,14, 369–379 (2015).
G. Ramos-Mandujano, R. Hernández-Benítez, and H. Pasantes-Morales, “Multiple mechanisms mediate the taurine-induced proliferation of neural stem/progenitor cells from the subventricular zone of the adult mouse,” Stem Cell Res.,12, 690–702 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorgani, S., Jahanshahi, M. & Elyasi, L. Taurine Prevents Passive Avoidance Memory Impairment, Accumulation of Amyloid-β Plaques, and Neuronal Loss in the Hippocampus of Scopolamine-Treated Rats. Neurophysiology 51, 171–179 (2019). https://doi.org/10.1007/s11062-019-09810-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-019-09810-y