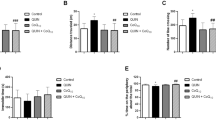
The paradigm of brain ischemic preconditioning (BIP) rendering protection from succeeding chemical insults has not been established in vivo. Systemic administration of 3-nitropropionic acid (3-NP) damages cerebral basal ganglia by inducing a mitochondrial dysfunction: this serves as a translational model for Huntington’s disease. We tested the potential of BIP against the neurotoxic effects of 3-NP and investigated the role of glycogen synthase kinase 3-beta (GSK-3β) and heat shock protein-72 (HSP72) signalling in the mentioned model. Male Wistar rats (n = 8; 180-200 g) were randomly assigned to seven groups, sham, BIP, 3NP, BIP+3NP, LiCl+BIP+3NP, Que (quercetin) +BIP+3NP, and LiCl+Que+BIP+3NP. Lithium chloride, 3-NP, and quercetin were administered in the doses of 40, 35, and 4 mg/kg i.p., respectively. Deficits in motor coordination and memory retention were assessed using the balance beam, accelerating rotarod, gait analysis, Morris water maze, and elevated plus maze. Brain biochemistry was assessed for the markers of lipid peroxidation and nitric oxide. The findings revealed noticeable differences in the measured indices in the examined animal groups vs. the sham group (P < 0.05 for motor coordination, memory retention, and brain biochemistry). A strong negative correlation between the rotarod performance of animals and their brain MDA levels was found. The findings reveal that BIP provides behavioral and biochemical recuperation against 3NP-induced neurodegeneration, and this is related to downregulation of GSK -3β and upregulation of HSP72.
Similar content being viewed by others
References
A. Durukan and T. Tatlisumak, “Preconditioning-induced ischemic tolerance: a window into endogenous gearing for cerebroprotection,” Exp. Transl. Stroke Med., 2, No. 2 (2010).doi: https://doi.org/10.1186/2040-7378-2-2.
A. Shaldubina, G. Agam, and R. H. Belmaker, “The mechanism of lithium action: state of the art, ten years later,” Prog. Neuropsychopharmacol. Biol. Psychiat., 25, No. 4, 855–866 (2001).
P. He, Y. Y. Fan, L. Y. Zhang, et al., “Effect of endogenous histamine on ischemic preconditioning induced cerebral ischemic tolerance,” Zhejiang Da Xue Xue Bao Yi Xue Ban, 38, No. 6, 579–583 (2009).
N. S. Thaddeus, “Animal models of global cerebral ischemia,” in Acute Stroke, Bench to Bedside, A. Bhardwaj, et al. (eds.), Informa Healthcare, New York (2006), pp. 275–292.
V. Borlongan, T. K. Koutouzis, T. S. Randall, et al., “Systemic 3-nitropropionic acid: Behavioral deficits and striatal damage in adult rats,” Brain Res. Bull., 36, No. 6, 549–556 (1995).
R. J. McDonald and N. M. White, “Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus,” Behav. Neural Biol., 61, No. 3, 260–270 (1994).
K. Ishiguro, A. Shiratsuchi, S. Sato, et al., “Glycogen synthase kinase 3β is identical to tau protein kinase I generating several epitopes of paired helical filaments,” FEBS Lett., 325, No. 3, 167–172 (1993).
R. J. Crowder and R. S. Freeman, “Glycogen synthase kinase-3β activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal,” J. Biol.Chem., 275, No. 44, 34266–34271 (2000).
P. Mendez, I. Azcoitia, and L. M. Garcia-Segura, “Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms,” J. Endocrinol., 185, No. 1, 11–17 (2005).
O. Varea, M. A. Arevalo, J. J. Garrido, et al., “Interaction of estrogen receptors with insulin-like growth factor-I and Wnt signaling in the nervous system,” Steroids, 75, Nos. 8/9, 565–569 (2010).
J. R. Woodgett, “Molecular cloning and expression of glycogen synthase kinase-3/factor A,” EMBO J., 9, No. 8, 2431–2438 (1990).
C. Hedgepeth, L. J. Conrad, J. Zhang, et al., “Activation of the Wnt signaling pathway: a molecular mechanism for lithium action,” Dev. Biol., 185, No. 1, 82–91 (1997).
F. R. Lucas, R. G. Goold, P. R. Gordon-Weeks, et al., “Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium,” J. Cell Sci., 111, Part 10, 1351–1361 (1998).
V. Stambolic, L. Ruel, and J. R. Woodgett, “Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells,” Current Biol., 6, No, 12, 1664–1668 (1996).
H. N. Yadav, M. Singh, and P. L. Sharma, “Pharmacological inhibition of GSK-3β produces late phase of cardioprotection in hyperlipidemic rat: possible involvement of HSP72,” Mol. Cell. Biochem., 369, Nos. 1/2, 227–233 (2012).
F. Zhang, C. J. Phiel, L. Spece, et al., “Inhibitory phos- phorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3,” J. Biol. Chem., 278, No. 35, 33067–33077 (2003).
V. L. Gabai, A. B. Meriin, D. D. Mosser, et al., “Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermo-tolerance,” J. Biol. Chem.,272, No. 29, 18033–18037 (1997).
A. R.Stankiewicz, G. Lachapelle, C. P. Foo, et al., “Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation,” J. Biol. Chem.,280, No. 46, 38729–38739 (2005).
M. Tanaka, H. Fujiwara, K. Yamasaki, et al., “Expression of heat shock protein after ischemic preconditioning in rabbit hearts,” Jpn. Circ. J., 62, No. 7, 512–516 (1998).
J. Jakubowicz-Gil, B. Pawlikowska-Pawlega, T. Piersiak, et al., “Quercetin suppresses heat shock-induced nuclear translocation of Hsp72,” Folia Histochem. Cytobiol., 43, No. 3, 123–128 (2005).
N. Nagai,A. Nakai, and K. Nagata, “Quercetin suppresses heat shock response by down regulation of HSF1,” Biochem. Biophys. Res. Commun., 208, No. 3, 1099–10105 (1995).
A. Debes, M. Oerding, R. Willers, et al., “Sensitization of human Ewing’s tumour cells to chemotherapy and heat treatment by the bioflavonoid quercetin,” Anticancer Res., 23, No. 4, 3359–3366 (2003).
A. Sharma and R. Goyal, “Experimental brain ischemic preconditioning: a concept to putative targets,” CNS Neurol. Disord. Drug Targets, 15, No. 4, 489–495 (2016).
L. B. Goldstein and J. N. Davis, “Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury,” J. Neurosci. Methods.,31, No. 2, 101–107 (1990).
M. Sharma and Y. K. Gupta, “Chronic treatment with trans-resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats,” Life Sci., 71, No. 21, 2489–2498 (2002).
E. D. Wills, “Mechanism of lipid peroxidase formation in animal tissue,” Biochem. J., 99, No. 3, 667–676 (1966).
L. C. Green, D. A. Wagner, J. Glogowski, et al., “Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids,” Anal. Biochem., 126, No. 1, 131–138 (1982).
K. Kitagawa, M. Matsumoto, K. Kuwabara, et al., “‘Ischemic tolerance’ phenomenon detected in various brain regions,” Brain Res., 561, No. 2, 203–211 (1991).
P. C. Severino, A. Muller Gdo, S. Vandresen-Filho, and C. I. Tasca, “Cell signaling in NMDA preconditioning and neuroprotection in convulsions induced by quinolinic acid,” Life Sci., 89, Nos. 15/16, 570–576 (2011).
K. B. Shpargel, W. Jalabi, Y. Jin, et al., “Preconditioning paradigms and pathways in the brain,” Cleve. Clin. J. Med., 75, Suppl. 2, S77–S82 (2008).
T. A. Alston, L. Mela, and H. J. Bright, “3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase,” Proc. Natl. Acad. Sci. USA, 74, No. 9, 3767–3771 (1977).
C. J. Coles, D. E. Edmondson, and T. P. Singer, “Inactivation of succinate dehydrogenase by 3-nitropropionate,” J. Biol. Chem.,254, No. 12, 5161–5167 (1979).
N. Bizat, J. M. Hermel, S. Humbert, et al., “In vivo calpain/caspase cross-talk during 3-nitropropionic acidinduced striatal degeneration: implication of a calpainmediated leavage of active caspase-3,” J. Biol. Chem., 278, No. 44, 43245–43253 (2003).
J. B. Schulz, R. T. Matthews, B. G. Jenkins, et al., “Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo,” J. Neurosci., 15, No. 12, 8419–8429 (1995).
E. Brouillet, C. Jacquard, N. Bizat, and D. Blum, “3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease,” J. Neurochem., 95. No. 6, 1521–1540 (2005).
S. Y. Shu, Y. M. Wu, X. M. Bao, and B. Leonard, “Interactions among memory-related centers in the brain,” J. Neurosci. Res., 71, No. 5, 609–616 (2003).
S. Y. Shu, G. Jiang, Q. Y. Zeng, et al., “The marginal division of the striatum and hippocampus has different role and mechanism in learning and memory,” Mol. Neurobiol., 51, No. 2, 827–839 (2015).
B. de Araújo Herculano, S. Vandresen-Filho, W. C. Martins, et al., “NMDA preconditioning protects against quinolinic acid-induced seizures via PKA, PI3K and MAPK/ErK signaling pathways,” Behav. Brain Res., 219. No. 1, 92–97 (2011).
T. P. Obrenovitch, “Molecular physiology of preconditioning- induced brain tolerance to ischemia,” Physiol. Rev., 88, No. 1, 211–247 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A., Goyal, R. Effects of Brain Ischemic Preconditioning on Cognitive Decline and Motor Incoordination in 3-Nitropropionic Acid-Intoxicated Rats: Probable Mechanisms of Action. Neurophysiology 51, 160–170 (2019). https://doi.org/10.1007/s11062-019-09809-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-019-09809-5