Abstract
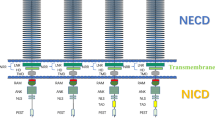
Notch signaling pathway, a highly conserved cell signaling system, exists in most multicellular organisms. The objective of this study was to examine Notch signaling pathway in germ cell cyst breakdown and primordial follicle formation. The receptor and ligand genes of Notch pathway (Notch1, Notch2, Jagged1, Jagged2 and Hes1) were extremely down-regulated after newborn mouse ovaries were cultured then exposed to DAPT or L-685,458 in vitro (P < 0.01). Since DAPT or L-685,548 inhibits Notch signaling pathway, the expression of protein LHX8 and NOBOX was significantly reduced during the formation of the primordial follicles. Down-regulated mRNA expression of specific genes including Lhx8, Figla, Sohlh2 and Nobox, were also observed. The percentages of female germ cells in germ cell cysts and primordial follicles were counted after culture of newborn ovaries for 3 days in vitro. The result showed female germ cells in cysts was remarkably up-regulated while as the oocytes in primordial follicles was significantly down-regulated (P < 0.05). In conclusion, Notch signaling pathway may regulate the formation of primordial follicle in mice.





Similar content being viewed by others
References
Kezele P, Nilsson E, Skinner MK (2002) Cell–cell interactions in primordial follicle assembly and development. Front Biosci 7:1990–1996
Pepling ME, Spradling AC (1998) Female mouse germ cells form synchronously dividing cysts. Development 125:3323–3328
Borum K (1961) Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res 24:495–507
Hirshfield AN (1991) Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101
Baker TG (1972) Gametogenesis. Acta Endocrinol Suppl (Copenh) 166:18–41
Liang L, Soyal SM, Dean J (1997) FIGLa, a germ cell specific transcription factor involved in the coordinate expression of the zona pellucid genes. Development 124:4939–4947
Soyal SM, Amleh A, Dean J (2000) FIGLa, a germ cell-specific transcription factor required for ovarian follicle formation. Development 127:4645–4654
Suzumori N, Yan C, Matzuk MM, Rajkovic A (2002) Nobox is a homeobox-encoding gene preferentially expressed in primordial and growing oocytes. Mech Dev 111:137–141
Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM (2004) NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science 305:1157–1159
Ballow DJ, Xin Y, Choi Y, Pangas SA, Rajkovic A (2006) Sohlh2 is a germ cell-specific bHLH transcription factor. Gene Expr Patterns 6:1014–1018
Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A (2006) Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA 103:8090–8095
Bruckner K, Perez L, Clausen H, Cohen S (2000) Glycosyltransferase activity of fringe modulates Notch–Delta interactions. Nature 406:411–415
Zhang CP, Yang JL, Zhang J, Li L, Huang L, Ji SY, Hu ZY, Gao F, Liu YX (2011) Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology 152:2437–2447
High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA (2007) An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest 117:353–363
IeM Shih, Wang TL (2007) Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res 67:1879–1882
Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD (1996) Truncated mammalian Notch1 activates CBF1/RBPJk-repressedgenes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol 16:952–959
Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A (1995) Signaling downstream of activated mammalian Notch. Nature 377:355–358
Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Gene Dev 12:2269–2277
Dumortier A, Wilson A, MacDonald HR, Radtke F (2005) Paradigms of notch signaling in mammals. Int J Hematol 82:277–284
Bray SJ (2006) Notch signaling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689
Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255
Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284:770–776
Zlobin A, Jang M, Miele L (2000) Toward the rational design of cell fate modifiers: notch signaling as a target for novel biopharmaceuticals. Curr Pharm Biotechnol 1:83–106
Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131:965–973
Revinski DR, Paganelli AR, Carrasco AE, López SL (2010) Delta-Notch signaling is involved in the segregation of the three germ layers in Xenopus laevis. Dev Biol 399:477–492
Dirami G, Ravindranath N, Achi MV, Dym M (2001) Expression of notch pathway components in spermatogonia and sertoli cells of neonatal mice. J Androl 22:944–952
Trombly DJ, Woodruff TK, Mayo KE (2009) Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150:1014–1024
Pan B, Chao HH, Chen B, Zhang LJ, Li L, Sun XF, Shen W (2011) DNA methylation of germ-cell-specific basic helix-loop-helix (HLH) transcription factors, Sohlh2 and Figlalpha during gametogenesis. Mol Hum Reprod 17:550–561
Chen B, Zhang LJ, Tang J, Feng XL, Feng YM, Liang GJ, Wang LQ, Feng YN, Li L, DeFelici M, Shi QH, Shen W (2013) Recovery of functional oocytes from cultured premeiotic germ cells after kidney capsule transplantation. Stem Cells Dev 22:567–580
Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W (2012) Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep 39:5651–5657
Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W (2013) Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen 54:354–361
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408
Zhang P, Chao HH, Sun SF, Li L, Shi QH, Shen W (2010) Murine folliculogenesis in vitro is stage-specifically regulated by insulin via the Akt signaling pathway. Histochem Cell Biol 134:75–82
Zhang ZP, Liang GJ, Zhang GL, Chao HH, Li L, Sun XF, Min LJ, Pan QJ, Shi QH, Sun QY, De Felici M, Shen W (2012) Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS ONE 7:e41771
Zhang LJ, Pan B, Zhang XF, Chen B, Liang GJ, Feng YN, Wang LQ, Ma JM, Shi QH, Shen W (2012) The characteristics of the expression and epigenetic modification of transcription regulator Lhx8 during oogenesis. Gene 506:1–9
SAS Institute (1996) SAS user’s guide: statistics, version 7.0 edn. SAS Institute, Cary
De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Schroeter EH, Mumm JS, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999) A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398:518–522
McGee EA, Hsueh AJ (2000) Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214
Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA (2006) Ovarian follicle development and transgenic mouse models. Hum Reprod Update 12:537–555
Visser JA, de Jong FH, Laven JS, Themmen AP (2006) Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 131:1–9
Knight PG, Glister C (2006) TGF-superfamily members and ovarian follicle development. Reproduction 132:191–206
Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J (2001) Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev 109:355–361
Sun LL, Sun ZY, Zhang P, Zhai XW, Tang J, Pan QJ, Shi QH, Shen W (2010) Effect of insulin on oogenesis from mouse fetal germ cells in a serum-free 3D culture system. Reprod Biomed Online 20:11–25
Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Kennedy SL, Johnson-Wood KL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Shopp GM, Seubert PA, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, Altstiel LD, May PC, Bender MH, Boggs LN, Britton TC, Clemens JC, Dieckman-McGinty DK, Czilli DL, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, John-stone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE (2001) Functionalγ-secretase inhibitors reduce β-amyloid peptide levels in brain. J Neurochem 76:173–181
Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Smith AL, Nadin A, Stevenson G, Castro JL (2000) L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid β-protein precursor γ-secretase activity. Biochemistry 39:8698–8704
Acknowledgments
This work was supported by National Nature Science Foundation (31001010, 31171376 and 31101716), National Basic Research Program of China (973 Program, 2012CB944401 and 2011CB944501), and Program for New Century Excellent Talents in University (NCET-12-1026), Foundation of Distinguished Young Scholars of Shandong Province (JQ201109), and Yantai Hi-Tech Zone Blue Ocean Talent Plan.
Conflict of interest
The authors fully declare any financial or other potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, CL., Fu, XF., Wang, LQ. et al. Primordial follicle assembly was regulated by notch signaling pathway in the mice. Mol Biol Rep 41, 1891–1899 (2014). https://doi.org/10.1007/s11033-014-3038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3038-4