Abstract
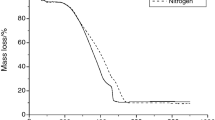
Three new energetic agents were synthesized using 3,5-dinitro-4-chlorobenzonitrile, sodium azide and hydrazine, which were 2,6-dinitro-4-cyano-azidobenzene (I), N-2,6-dinitro-cyanophenyl-hydrazine (II) and bis-N,N′(2,6-dinitro-4-cyanophenyl)hydrazine (III). These energetic substances were first characterized by elemental analysis, IR, mass, 1H NMR and 13C NMR spectroscopic methods. The energetic substances were studied by thermogravimetry, and it was understood that the mechanism of the thermal decomposition reactions consists of two successive exothermic thermal reactions. In the first thermal reaction, the energetic material was converted to furoxane compounds, and then, these furoxane compounds were decomposed by the second thermal reaction. Activation energies and Arrhenius pre-exponential factors of thermal responses were determined by using isothermal (Coats–Redfern) and nonisothermal/isoconvertional (Kissinger–Akahira–Sunose, Ozawa–Flynn–Wall) methods with thermogravimetry and differential scanning calorimetry (DSC) data. With these calculated values, other thermodynamic parameters reaction enthalpy, entropy changes and free energy were calculated. Formation enthalpies of the elements of the energetic substances were theoretically calculated using the CBS-4M algorithm in the Gaussian 09 program for the synthesized energetic substances. In the thermal decomposition reactions, the products were estimated with the aid of literature data and the enthalpies of explosion reactions were theoretically calculated according to the Hess Law. Besides, the exothermic energies in the first and second thermal reactions of the energetic substances were measured by DSC. The results measured by DSC were compared with the calculated theoretical results and were found to be very close to each other. In the study, antimicrobial activity was estimated to be high because energetic molecules are strained molecules, and it is possible this tension can affect the medium. According to this thought, antimicrobial activity was determined by using five different bacteria and a fungus. Antimicrobial activity values were determined by “agar dilution” method, and results were found as minimum inhibition concentration. Among the three energetic substances, 2,6-dinitro-4-cyano-azidobenzene was found to have the most active compound.









Similar content being viewed by others
References
Agrawal JP, Hodgson RD. Organic chemistry of explosives. Sussex: John Wiley and Sons; 2006. p. 158–62.
Agrawal JP, Surve RN, Mehilal Sonawane SH. Some aromatic nitrate esters: synthesis, structural aspects, thermal and explosive properties. J Hazard Mater. 2000;77:11–31.
Agrawal JP. High energy materials. Weinheim: Wiley; 2010. p. 93.
Badgujar DM, Talawar MB, Harlapur SF, Asthana SN, Mahulikar PP. Synthesis, characterization and evaluation of 1,2-bis(2,4,6-trinitrophenyl) hydrazine. J Hazard Mater. 2009;172:276–9.
Yiğiter AÖ, Atakol MK, Aksu ML, Atakol O. Thermal characterization and theoretical and experimental comparison of picryl chloride derivatives of heterocyclic energetic compounds. J Therm Anal Calorim. 2017;127:2199–213.
Atakol M, Atakol A, Yiğiter AÖ, Svoboda I, Atakol O. Investigation of energetic materials prepared by reactions of diamines with picryl chloride: synthesis, structure and thermal behaviour. J Therm Anal Calorim. 2017;127:1931–40.
Klapötke TM. Chemsitry of high-energy materials. Berlin: Walter de Gruyter; 2012. p. 141–64.
Politzer P, Murray JS. Energetic materials part 1. Decomposition, crystal and molecular properties. Amsterdam: Elsevier; 2003. p. 411–6.
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP. Advances in science and technology of modern energetic materials: an overview. J Hazard Mater. 2008;151:289–305.
Bailey AS, Case JR. 4:6-dinitrobenzofuroxan, nitrobenzodifuroxan and benzotrifuroxan: a new series of complex-forming reagents for aromatic hydrocarbons. Tetrahedron. 1958;3:113–31.
Reddy GO, Murall BKM, Chotterjee AK. Thermal study on picryl azide (2-azido-1,3,5-trinitrobenzene) decomposition using simultaneous thermogravimetry and differential scanning calorimetry. Propellant Explos Pyrotech. 1983;8:29–33.
Shremetev AB, Aleksandrova NS, Ignat NV, Schulte M. Straightforward one-pot synthesis of benzofuroxans from o-halonitrobenzenes in ionic liquids. Mendeleev Commun. 2012;22:95–7.
Cardillo P, Gigante L, Lunghi A, Zanirato P. Revisiting the thermal decomposition of five ortho-substituted phenyl azides by calorimetric technics. J Therm Anal Calorim. 2010;100:191–8.
Fu XL, Fan XZ, Wang BZ, Huo H, Li JZ, Hu RZ. Thermal behavior, decomposition mechanism and thermal safety of 5,7-diamino-4,6-dinitrobenzofuroxan (CL-14). J Therm Anal Calorim. 2016;124:993–1001.
Özkaramete E, Şenocak N, İnal EK, Öz S, Svoboda I, Atakol O. Experimental and computational studies on the thermal degradation of nitroazidobenzenes. Propellant Explos Pyrotech. 2013;38:113–9.
Atkins P, Paula JD. Atkin’s physical chemistry. 8th ed. Oxford: Oxford University Press; 2006. p. 45.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetrical data. Nature. 1964;201:68–9.
Ebrahimi HP, Hadi JS, Abdulnabi ZA, Bolandnazar Z. Spectroscopic, thermal analysis and DFT computational studies of Salen type Schiff base complexes. Spectrochim Acta Part A. 2014;117:485–92.
Abdel-Kader NS, Amin RM, El-Ansary AL. Complexes of Schiff base of benzopyran-4-one derivative. J Therm Anal Calorim. 2016;123:1695–706.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Koga N. Ozawa’s kinetic method for analysing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Popescu C, Sbirazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Çılgı GK, Çetişli H, Donat R. Thermal kinetic analysis of uranium salts. J Therm Anal Calorim. 2014;115:2007–20.
Kullyakool S, Danvirutai C, Siriwong K, Noisong P. Determination of kinetic triplet of the synthesized Ni3(PO4)2·8H2O by non-isothermal and isothermal kinetic methods. J Therm Anal Calorim. 2014;115:1497–507.
Sarada K, Muraleedharan K. Effect of addition of silver on the thermal decomposition kinetics of copper oxalate. J Therm Anal Calorim. 2016;123:643–51.
Ledeti I, Fuliaş A, Vlase G, Vlase T, Bercean V, Doca N. Thermal behaviour and kinetic study of some triazoles as potential anti-inflammatory agents. J Therm Anal Calorim. 2013;114:1295–305.
Zianna A, Vecchio S, Gdaniee M, Czapik A, Hadzidimitriou A, Lalia-Kantouri M. Synthesis, thermal analysis and spectroscopic and structural characterizations of zinc(II) complexes with salicylaldehydes. J Therm Anal Calorim. 2013;112:455–64.
Gaussian 09, Revision D.01. Gaussian Inc. Wallingford CT, USA, 2009.
Ochterski JW, Petersson GA, Montgomery JA Jr. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J Chem Phys. 1996;104:2598–619.
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA. A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys. 2000;112:6532–42.
Gökçınar E, Klapötke TM, Bellamy AJ. Computational study on 2,6-diamino-3,5-dinitropyrazine and its 1-oxide and 1,4-dioxide derivatives. J Mol Struct. 2010;953:18–23.
Politzer P, Lane P, Concha MC. Computational determination of nitroaromatic solid phase heats of formation. Struct Chem. 2004;15:468–79.
Chiato ZL, Klapötke TM, Mieskes F, Stierstorfer J, Weyrauther M. (Picrylamino)-1,2,4-triazole derivatives-thermal stabl explosives. Eur J Inorg Chem. 2016;956-62.
Nist Chemistry WebBook, webbook. nist. gov/chemistry.
National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. VIth informational supplement. M100S9 National Committee for Clinical Laboratory Standards, Villanova, PA. 1999.
Coyle MB. Manual of antimicrobial susceptibility testing, MIC testing. Washington: American Society for Microbiology; 2005. p. 53–62.
Hadacek F, Greger H. Testing of antifungal Natural products, methadologies, comparability of results and assay choice. Phytochem Anal. 2000;11:137–47.
Şen N, Özkaramete E, Yılmaz N, Öz S, Svoboda IM, Akay A, Atakol O. Thermal decomposition of dinitro-chloro-azido benzenes: a comparison of theoretical and experimental results. J Energy Mater. 2014;32:1–15.
Sarlauskas J, Anusevicius Z, Misiunas A. Benzofuroxan (Benzo[1,2-c]1,2,5-oxadiazole N-oxide) derivatives and potential energetic materials. Central Eur J Energy Mater. 2012;9:365–86.
Klapötke TM. Chemsitry of high-energy materials. Berlin: Walter de Gruyter; 2012. p. 75–6.
Kubota N. Propellants and explosives. 2nd ed. Weinheim: Wiley; 2007. p. 36.
Liu QR, Xue LW, Zhao GQ. Manganese(III) complexes derived from bis-Schiff Bases. Russ J Coord Chem. 2014;40:757–63.
Montazerozohari M, Jahromi SM, Masoudiasl A, McArdle P. Nanostructure zinc(II) Schiff Base complexes of a N-3-tridentate ligand as new biological active agents. Spectrochim Acta A. 2015;138:517–28.
Gaelle DSY, Yufanyi DM, Jagan R, Agwara MO. Synthesis, characterization and antimicrobial activity of cobalt(II) and cobalt(III) complexes derived from 1,10-phenantroline with nitrate and azide co-ligands. Cogent Chem. 2016; Article number: 1253201; doi: 10.1080/23312009.2016.1253201.
Acknowledgements
This work was supported by the Scientific Research Fund of the University of Ankara (project no. 16H0430004) and Scientific Research Fund of the Ahi Evran University (grant no: FEF.A4.17.001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sopacı, Ş.B., Nazır, H., Emir, E. et al. Thermal kinetic analysis, theoretical thermodynamic calculations and antimicrobial activity of three new energetic materials. J Therm Anal Calorim 131, 3105–3120 (2018). https://doi.org/10.1007/s10973-017-6708-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6708-3