Abstract
The aim of the presented research was to evaluate the antimicrobial, anti-tuberculosis and antioxidant activity of thiosemicarbazone derivatives. The thermal stability and thermal degradation of nineteen compounds were determined. The results obtained in in vitro tests indicate the potential of selected compounds against Staphylococcus aureus, Staphylococcus epidermidis and Mycobacterium tuberculosis. Some compounds turned out to be active free radical scavengers and at the same time Fe3+ reducing agents. The performed TGA, and c-DTA measurements showed different thermal stable of thiosemicarbazone derivatives. The T2 derivative was the most thermally resistant. On the other hand, the T11 derivative was the least resistant. The performed thermal analysis showed that most of the derivatives underwent two-stage thermal decomposition (13 samples).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thiosemicarbazones are a group of compounds usually obtained by the condensation of a thiosemicarbazide with an aldehyde or ketone [1]. In the context of biological activity, complexes of thiosemicarbazones with various metals seem to be of particular interest [2,3,4]. Hybrid thiosemicarbazone-copper complexes have been shown to have development potential as new anticancer agents [5, 6]. Copper(II) complexes displayed antiproliferative activity in CH1 ovarian carcinoma cells and inhibited Topoisomerase IIα activity in a DNA plasmid relaxation assay [7]. The Ru (II) steroid complexes inhibit the growth of gram-positive and gram-negative bacteria [8].
However, for many years the attention of scientists has been focused on thiosemicarbazone derivatives not only as potential ligands but also as compounds with a broad spectrum of biological activity [9,10,11,12]. Over the past decades, thiosemicarbazones have been clinically developed for a variety of diseases, including tuberculosis, viral infections, malaria and cancer [13]. Some thiosemicarbazone derivatives significantly inhibited the growth of Hep3B, A549 and MCF-7 tumor cells [14]. Tyrosinase-inhibiting thiosemicarbazones may find application in the development of anti-melanogenetic agents for skin whitening cosmetics and anti-bronze agents for food [15].
The thiosemicarbazones containing adamantane backbones showed good antifungal activity against Candida albicans, Enterococcus faecalis, Staphylococcus aureus and Bacillus cereus. The thiosemicarbazone derivatives obtained by reacting 4-[4-(trifluoromethyl)phenyl] thiosemicarbazide with aromatic aldehydes were more effective against Enterococcus faecalis than chloramphenicol. Furthermore, these compounds exhibited the same level of antifungal activity against Candida glabrata as ketoconazole [16]. On the other hand, thiosemicarbazones and N,N-dimethylthiosemicarbazones showed strong growth inhibition of Mycobacterium tuberculosis H37Rv [17]. Therefore, thiosemicarbazone derivatives can be used as model compounds for the design of substances with antimicrobial activity.
In the previous work, the obtained thiosemicarbazone derivatives were complexed and their anti-tumor potential was investigated [18]. This work investigated the antimicrobial and antioxidant activity of thiosemicarbazone derivatives. Moreover, for the sake of the potential biomedical application, the thermal stability of the compounds obtained was determined. A very important aspect of pre-formulation studies of new active pharmaceutical ingredients (API) is their physicochemical evaluation [19, 20]. One of the stages are research with the use of thermal analysis (TGA, DTA, DSC) [21]. They make it possible to determine the thermal resistance of new substances. This is of great importance, especially when we want to determine the appropriate storage conditions for new drugs [22, 23]. In addition, thermal analysis is widely used in pharmacy [24,25,26,27]. Many works concern its application, among others, in the assessment of the compatibility of API and excipients [24], substance polymorphism [25], moisture content [26] or assessment of sterilization conditions [27].
Experimental
The title compounds were synthesized according to the following procedure: the mixture of thiosemicarbazide (0.01 mol) and aldehyde or ketone (0.01 mol) in anhydrous ethanol (20 cm3) was heated to reflux for 0.5–1 h. Final compounds were obtained after cooling the mixture to room temperature. Purification was done by crystallization from ethanol. The detailed physicochemical and spectral properties of the investigated compounds were described in the previous paper [18]. All chemicals used for the synthesis were purchased from the companies Sigma-Aldrich, AlfaAesar, POCH and used without further purification.
Methods and physical measurements
Antimicrobial studies
Antimicrobial activities of the compounds were tested in vitro against reference Gram-positive cocci (S. aureus: ATCC 25,923, NCTC 4163, ATCC 29,213, ATCC 6538, S. epidermidis: ATCC 35,984, ATCC12228, Enterococcus hirae ATCC 10,541) and Gram-negative rods (E. coli: ATCC 10,538, ATCC 25,922, P. aeruginosa: ATCC 15,442). Minimal Inhibitory Concentration (MIC) of tested compounds and the reference antibacterial drug—Ciprofloxacin, were examined by the twofold serial broth dilution methods in Mueller–Hinton Broth medium using 96-well microtiter plates (Medlab Products) according to CLSI guidelines [28]. Concentrations of tested compounds in liquid medium ranged from 256 to 0.125 µg cm−3. The final inoculum of all microorganisms studied was approximately 105 cfu cm−3. MIC for bacteria was defined as the lowest drug concentration that completely inhibits growth of the microorganism in the microdilution wells as detected by the unaided eye after 18 h of incubation at 35 ºC. Ciprofloxacin was used as reference compound.
Antitubercular studies
The tuberculostatic activity of compounds was tested in Department of Microbiology, National Tuberculosis and Lung Diseases Research Institute, Warsaw. The synthesized compounds were examined in vitro for their tuberculostatic activity against the M. tuberculosis H37Rv strain (ATCC 25,618) and two ‘wild’ strains isolated from tuberculosis patients: one (Spec. 210) resistant to p-aminosalicylic acid (PAS), isonicotinic acid hydrazide (INH), etambutol (ETB) and rifampicin (RMP) and the another (Spec. 192) fully sensitive to the administrated tuberculostatics. Investigations were performed by broth microdilution method according to CLSI M24, 3rd ed. [29]. Investigations were performed in 96-well microliter plates by the twofold serial microdilution using Middlebrook 7H9 Broth medium (Beckton Dickinson) containing 10% of OADC (Beckton Dickinson). The inoculum was prepared from fresh LJ culture in Middlebrook 7H9 Broth medium with OADC, adjusted to a no. 0.5 McFarland tube, and diluted 1:100. The stock solution of tested agent was prepared in DMSO. Each test compound stock solutions was diluted in Middlebrook 7H9 Broth medium with OADC by four-fold the final highest concentration to be tested. Compounds were diluted serially in a sterile 96-well microtiter plates using 100 μl Middlebrook 7H9 Broth medium with OADC. Concentrations of tested agents ranged from 512 to 0.0625 µg cm−3. A growth control containing no antibiotic and a sterile control without inoculation were also prepared on each plate. The plates were incubated at 37 °C for 21 days. After incubation period, 30μL of Alamar blue solution was added to each well, and the plate was re-incubated for 24 h. Growth is indicated by the a color change from blue to pink and the lowest concentration of compound that prevented the color change was noted as its MIC [30, 31]. Isoniazid (INH) as reference drug was used for comparison.
Antioxidant studies
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, TPTZ (2,4,6-Tri(2-pyridyl)-s-triazine)) and trolox (( ±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, 97%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Acetic acid (ACS), FeCl3 × 6H2O, FeSO4 × 7H2O, phosphoric acid (ACS), hydrochloric acid (ACS) and methanol was purchased from Polish Reagents (Gliwice, Poland). Absorbance was measured by a UV–Vis spectrophotometer Genesis 20 (Thermo FisherScientific, Waltham, MA, USA) in a 1 cm quartz cell.
DPPH
Antioxidant activity was determined with use of colorimetric method based on stable free radicals DPPH. The solution of free radicals was prepared by mixing 0.004 g DPPH in 100 cm3 of methanol. The reagent was prepared prior analysis. Free radical scavenging activity of analyzed compounds was determined in form of percentage scavenging of free radicals as well as IC50 value (concentration providing 50% free radical scavenging). The IC50 was determined for compounds which revealed above 50% activity at concentration equal 100 μg cm−3 after 15 min from reaction initiation. In order to obtain IC50 value, the following concentrations of compounds were used [μg cm−3]: 3.12; 12.5; 25; and 100. The appropriate amount of compounds dissolved in methanol (T1, T2, T3, T4, T6, T7, T11, T13, T14, T18, T19) or DMSO (T5, T8, T9, T10, T12, T15, T16, T17) were added to 3 mL of DPPH solution. The absorbance was measured directly after mixing the substances and absorbance changes were recorded after each 5 min up to 30 min. Trolox was used as standard antioxidant. All measurements were repeated three times. Free radical scavenging activity was calculated with the use of the following formula:
where, Ablank is the absorbance of the sample except tested substances and Asample is the absorbance of the sample with tested compounds.
FRAP assay
FRAP assay was performed based on [32] with some modifications: FRAP solution was prepared freshly each time by mixing (10:1:1 v/v/v) 0.3 M acetate buffer (pH 3.6), 0.01 M TPTZ in 0.04 M HCl and 0.02 M FeCl3 × 6H2O and kept in dark. An appropriate amount of studied compounds dissolved in methanol or DMSO [see: DPPH methodology] were added to 3 cm3 of FRAP solution. In order to maintain a constant dilution, the samples were made up to a volume of 3.155 mL with methanol or DMSO. The prepared samples were vortexed and incubated at 37 °C by 30 min away from light. Thereafter, absorbance was measured at 593 nm using Genesys 20 UV−Vis spectrophotometer in a 1 cm quartz cell. The assay was performed for the following sample concentrations [µg cm−3]: 3.12 and 25. All measurements were carried out in triplicate. The results were calculated into Fe2+ [µg cm3] on the calibration curve which was prepared analogically using aqueous solution of FeSO4 at concentration 1–5 µg mL−1 (y = 0.3724x + 0.0362, R2 = 0.9982). Trolox was used as standard reducer.
Thermal analysis
The thermal stability of tested samples (T1-T19) was determined by thermogravimetric analysis. Thermogravimeter TG 209 F3 Tarsus produced by Netzsch (Germany) was used in the research. For tested samples, thermogravimetric dynamic measurements were performed. For dynamic measurements, the TG, DTG, and c-DTA curves were recorded for 5 mg of tested samples at a heating rate of 10 K min−1. in the temperature range of 35–600 °C under N2 atmosphere. For analysis, the total flow nitrogen rate was the 40 mL min−1. Al2O3 crucible type used for measurement.
Calculated DTA (c-DTA) for endothermal and exothermal effects were performed for the samples. In this method the multiple-point temperature calibration was carried out by means of c-DTA. For this purpose the onset temperatures of the melting peaks of high-purity reference materials (In, Sn, Zn, Al, BaCO3, and Au) over the entire temperature range were performed.
The samples curves were analyzed using Proteus 8.0 software produced by Netzsch Company (Germany).
Results and discussion
In this study, thiosemicarbazone derivatives were obtained using the classical condensation reaction of thiosemicarbazide derivatives with selected carbonyl compounds. The following thiosemicarbazides were used for the reaction: 4-methylthiosemicarbazide, 4-phenyl-thiosemicarbazide, 2-methylphenylthiosemicarbazide, 2-chlorophenylthiosemicarbazide,
3-chlorophenylthiosemicarbazide. The thiosemicarbazides were heated with the appropriate aldehyde (4-bromobenzaldehyde, 3,4-dimethoxybenzaldehyde, 3,4-dichlorobenzaldehyde) or ketone (4-aminoacetophenone, acetophenone). The substrates were heated to the boiling point of the alcohol. Thus, nineteen compounds from the group of thiosemicarbazone derivatives (T1-T19) were obtained. The compounds were characterized on the basis of spectroscopic methods and described in the publication Pitucha et al. [18].
The article describes the thermal stability of the compounds obtained and their biological potential. The structures of investigated compounds are shown in Fig. 1.
The aim of the antimicrobial studies was to determine the value of the minimum inhibitory concentration (MIC). The range of tested concentrations for the MIC values was 256–0.125 µg cm−3. The reference in the studies was ciprofloxacin. Data for the most promising substances are presented in Table 1. The MIC values of the antimicrobial activity for all thiosemicarbazone derivatives are given in Table 1S (supplementary material).
The obtained data show that the tested compounds show a medium potential for antibacterial activity against gram-positive bacteria. The strongest inhibitory effect on the growth of S. aureus and S. epidermidis (ATCC 12,228) was demonstrated by the compound T7 (MIC = 64 µg cm−3) and the compound T8 against S. aureus (NCTC 4163) and S. epidermidis at the same concentration. Unfortunately, the remaining newly obtained thiosemicarbazone derivatives did not show any antibacterial activity against the tested gram-negative bacteria and none of the derivatives showed the same activity as the activity of the standard—ciprofloxacin.
The next step in the evaluation of the potential for antimicrobial activity of the obtained thiosemicarbazone derivatives was to select the derivatives in terms of their antimicrobial activity. The measure of effectiveness was the determination of the MIC value of pathogenic microorganisms. The tests were performed on three selected strains: M. tuberculosis H37Rv, M. tuberculosis sensitive to INH (strain no. 192), M. tuberculosis resistant to INH (strain no. 210). The MIC values for the most active thiosemicarbazone derivatives are presented in Table 2. The MIC values of the antimycobacterial activity for all thiosemicarbazone derivatives are given in Table 2S (supplementary material).
Tests in vitro performed for all compounds indicated the potential of several derivatives. Compound T2 inhibited the growth of all pathogens at a concentration of 128 µg cm−3. The most interesting results are for the substances T11 and T19. These compounds inhibited the growth of mycobacteria already at a concentration of 8 and 4 µg cm−3, respectively, for all tested strains. The obtained MIC values were lower than for the standard (Isoniazid). For the remaining compounds, the MIC values obtained were above 128 µg cm−3.
One of the most often studied biological activity is ability to scavenge free radicals as well as antioxidant activity. The phenomenon results from the fact that free radicals, the highly reactive molecules, are able to attack membranes or other parts of cell leading to their damage. It is known that they can oxidize amino acids, unsaturated fatty acids and other compounds being crucial for proper functioning organism [33, 34]. Oxidative stress, being consequence of imbalance between free radicals and their scavengers in organism, leads to various disorders, including neurodegeneration, cancer, and cardiovascular diseases. Similarly important issues for our organism are metal ions, especially so-called essential metals indispensable for health organism. Examples can be iron, copper or zinc ions which are responsible for correct functioning of enzymes (e.g., catalyze, SOD1, NADH hydrogenase) [35]. Besides the benefit properties of trace metal ions, they can catalyze oxidative reaction leading to free radicals, thus balance between positive and negative influence of the structures are a very thin line. Searching for compounds revealing ability to reduce trace metal ions to their less toxic form is highly desirable.
Antioxidant activity was determined with use of colorimetric method based on stable free radicals DPPH. The highest free radical scavenging activity was observed for the following compounds: T4, T11, T14, T15, T16 and T19. All of them revealed activity above 50% at concentration 100 μg cm−3 after 15 min from reaction initiation. Among them the highest activity was observed for T4 (93.97%) whereas the lowest activity revealed T16 (60.50%). The remaining compounds revealed significantly lower or lack ability to scavenge free radicals due to their activity was found to be below the 50% threshold (Table 3). Additional parameters, such as trolox equivalent and it modifications were determined for all samples whereas IC50 value was calculated for the most active samples. The following compounds: T4, T11, T13, T15, T19 can be discussed as the most prominent free radical scavengers. The high activity was observed for activity – concentration dependency (Fig. 2).
The highest free radical scavenging activity was observed for the following compounds: T4, T11, T14, T15, T16 and T19. All of them revealed activity above 50% at concentration 100 μg cm−3 after 15 min from reaction initiation. Among them the highest activity was observed for T4 (93.97%) whereas the lowest activity revealed T16 (60.50%). The remaining compounds revealed significantly lower or lack ability to scavenge free radicals due to their activity was found to be below the 50% threshold (Table 3). Additional parameters such as trolox equivalent and it modifications were determined for all samples whereas IC50 value was calculated for the most active samples. The following compounds: T4, T11, T13, T15, T19 can be discussed as the most prominent free radical scavengers. The high activity was observed for activity – concentration dependency (Fig. 2).
In the pooled results, T4, T11 and T19 can be regarded as the most active antioxidants, due the compounds revealed the greatest concentration – free radical scavenging dependency and trolox equivalent values (Fig. 2). The remaining compounds also revealed satisfactory activity nevertheless the aforementioned values were slightly lower.
In order to evaluate ability of selected compounds to reduction of Fe3+, ferric ion reducing antioxidant parameter (FRAP) method was used. Similarly to DPPH, some of studied compounds revealed high activity which is presented as Fe2+ concentration and trolox equivalent concentration. Analyzing output of the method, the most active turned out to be: T4, T7, T11, T14 and T19 which revealed high reduction ability at both concentrations (25 and 3.12 µg cm−3] (Table 4). Similarly to DPPH, the most prominent antioxidants able to reduction of metal ions turned out to be T4, T11 and T19, due to all of them revealed the highest Fe2+ concentration in the result of reduction reaction and trolox equivalent concentration at lowest sample concentration equal 3.12 µg cm−3. In some cases, absorbance out of scope (above 3.000) was recorded what indicates on high activity of samples but simultaneously the samples at concentration 3.12 µg cm−3 revealed cloudiness or low Fe2+ concentration (T1, T15, T16, T17). Additionally, in the case of T8 and T12 false negative results were recorded.
Considering the obtained results, a few active free radical scavengers and simultaneously Fe3+ reducers can be found. Among them the most promising are T4, T11 and T19 whereas T7, T13, T14, T15 and T16 which revealed at least one of the aforementioned activities. It is worth to mention, that high free radical scavenging was observed immediately after reaction initiation even at the lowest concentration. In reference to standard antioxidant—trolox, IC50 of the most active compound (T4) revealed 23.32% (Table 4) of trolox equivalent activity what is highly satisfactory result. The features of compounds indicate on the fact that can be considered as substances counteracting oxidative changes in organism and simultaneously protect it against various diseases which development is closely related to oxidative stress.
Thermal analysis
Analysis in the temperature range investigated the TGA curves of all tested samples showed differences in their stability. The most temperature stable sample is T2. For this sample onset decomposition temperature is 221.6 °C (Table 5). In turn, the least temperature stable sample is T11. This sample was stable up to 154.2 °C (Table 5). Taking into account the onset temperature of the decomposition processes, the thermal stability has got the following order: T11 < T19 = T4 < T7 < T1 = T13 < T14 < T6 < T18 < T10 < T12 < T9 = T16 < T8 < T3 < T15 < T17 < T5 < T2.
According to the TG, and 1st derivative of the TG curves the thermal decomposition process for tested samples was accompanied by one (T5), two (T1-T4, T6, T8, T10-T12, T15-T17, T19), three (T7, T13, T14, T18), and four stage (T9) mass loss (Table 6). Sample T5 completely degrades the compound in one step. The thiosemicarbazone derivatives chain is probably broken in the first stage samples that undergo two- and three-step degradation. In the next stages, the decomposition of individual components takes place. The decomposition of sample T3 is similar to the other samples undergoing multi-stage degradation, except that water molecules adsorbed in the crystalline solid are released in the first stage [36].
Endothermic and exothermic events were observed in the range of 93.4 °C for T4 to 218.3 °C, 218.4 °C for T17, T15 (Table 7). That events were related to the melting of samples (endo-effect), and their degradation (exo-effect) [37]. Taking into account the melting point of the tested samples, it is in the following order: T4 < T11 < T9 < T19 < T7 < T13 < T14 < T1 < T3 < T6 < T18 < T10 < T2 < T5 < T16 < T8 < T12 < T17 = T15 (Table 7). The recorded sharp endothermic peaks on the c-DTA curves indicate the high purity of the synthesized samples [38].
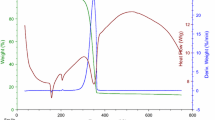
The TG/DTG, and c-DTA curves for selected samples are presented in Fig. 3. TG, DTG, and c-DTA parameters of curves obtained for samples (T1-T19) are shown in Tables 5–7, respectively.
Thermogravimetric curve of T5 sample presented in Fig. 3a showed that the thermally decomposition was stated at 214.3 °C, and contain one-stage. The DTG curve of T5 presented one peak corresponding with TG curve (Fig. 3a). DTG I stage of mass loss occurred in temperature range 188 °C to 403 °C with maximum peak in 258.9 °C. This stage is associated with a degradation of sample, and corresponding with exothermic peak in c-DTA curve (Fig. 3a, Table 7).
Figure 3b presents TG curve of a selected sample (T10) for example, from samples which undergo two-stage decomposition. TG analysis indicated that the decomposition of T10 is started in 194.4 °C and has got II stages of mass loss (Fig. 3b, Table 5). The first and second stage of mass loss is 40.2%, and 40%, respectively. The DTG curve of T10 presented two peaks corresponding with TG curve (Fig. 3b, Table 6). The first stage of mass loss occurred in temperature range 174 °C to 230 °C with maximum peak in 202.9 °C. This step corresponded with endothermic peak (198.2 °C) recorded in c-DTA curve, and was related with melting point of sample (Fig. 3b, Table 7). The second stage occurred in temperature range 230 °C to 380 °C with maximum peak in 299.1 °C and it was associated with a thermal degradation of the sample (Fig. 3b, Table 6). This stage corresponded with exothermic peak in c-DTA curve (Fig. 3b, Table 7).
Termogravimetry curve of T14 sample showed that the onset of thermally decomposition is 184.3 °C (Fig. 3c, Table 6), and corresponded with endothermic peak recorded in c-DTA curve (183.9 °C) (Fig. 3c. Table 7). This sample (T14) was selected, for example, from samples undergoing three-stage decomposition. In Fig. 3c we can observe III stages of mass loss. The first stage of mass loss is 37%, the second stage of mass loss is 10.6%, and the third stage of mass loss is 29.5%. The DTG curve showed in Fig. 3c presented three peaks corresponding with TG curve. The first stage of mass loss occurred in the temperature range 158 °C to 223 °C with maximum peak in 195.3 °C. The second stage of mass loss occurred in the temperature range 223 °C to 259 °C with maximum peak in 246.8 °C. The third stage of mass loss occurred in the temperature range 259 °C to 378 °C with maximum peak in 299.4 °C. For T14 sample exothermic peak in 220.7 °C on c-DTA curve was recorded (Fig. 3c, Table 7). This peak was related with the thermal decomposition of sample.
Figure 3d shows only one of the tested samples that undergoes four-stage decomposition. The DTG curve of T9 sample presented four peaks corresponding with TG curve (Fig. 3d, Table 6). The first stage of mass loss occurred in temperature range 103 °C to 177 °C with maximum peak in 149.6 °C. This stage is related with water release which is confirmed by the endothermic peak in 142.5 °C registered on c-DTA curve [39]. The second stage occurred in temperature range 179 °C to 234 °C with maximum peak in 214.1 °C, and is associated with the melting point of the tested sample. This fact was confirmed by the endothermic peak in 212.9 °C recorded on c-DTA curve (Fig. 3d, Table 7). The third stage occurred in temperature range 234 °C to 305 °C with maximum mass change in 237 °C, and is associated with a decomposition of the sample. This fact was confirmed by the exothermic peak (234.7 °C) on c-DTA curve (Fig. 3d, Table 7). The fourth stage begins in temperature 305 °C, and is associated with a continue degradation of the sample. The maximum mass loss peak for the last stage is in 315.8 °C.
From the conducted analysis, we can observe that the substitution of the methyl group (CH3) in the R1 position causes the decomposition of the compound at lower temperatures (< 160 °C). This is evident for the compounds T4, T11 and T19.
All samples have a major mass loss (5.79%-26.19%) occurring in the first stage, which a maximum peak temperature of 169.5 °C-258.9 °C (Table 6). Sample T9 behaves differently, with the maximum loss in the second stage (19.92%, 214.1 °C). This can be a result of a release of hydrated water molecules in the crystalline solid in the first stage [36, 39]. This theory is confirmed by the broad exothermic peak with a maximum temperature of 142.5 °C recorded on the c-DTA curve (Fig. 3a, Table 7).
On the other hand, we can see that the substitution of the dichlorophenyl (3,4-diClC6H3) group in the R2 position and the chlorophenyl (4-ClC6H4) group in the R position increases the onset temperature of thermal decomposition. In addition, we can see that for compounds having a dichlorophenyl (3,4-diClC6H3) group in the R2 position, the onset temperature of decomposition increases for the chlorophenyl (ClC6H4) substituents in the R position in order for the location of the chlorine substituent, i.e., T17 (4-Cl) > T15 (3-Cl) > T12 (2-Cl).
Also, the substitution at the R2 position of bromophenyl (4-BrC6H4) increases the thermal stability of the compounds. Generally, among the analyzed compounds, the compounds with substituted halogenophenyl derivatives (Br, Cl) in the R2 position increase the thermal stability of the tested derivatives. This may point to the potentially best derivatives in terms of stability as potential future drugs.
Conclusions
The thiosemicarbazone derivatives show a moderate antimicrobial potential. The strongest inhibition of S. aureus and S. epidermidis growth was observed for the compounds T7 and T8.
Two compounds inhibited the growth of mycobacteria already at a concentration of 8 and 4 µg cm−3, respectively, for all strains tested. The obtained MIC values were lower than for the standard (isoniazid). Some compounds turned out to be active free radical scavengers and at the same time Fe3+ reducing agents. Among them, the T4, T11 and T19 are the most promising. The IC50 of the most active compound (T4) showed a satisfactory activity equivalent to that of trolox. The performed thermal analyzes shown different thermal stable of tested thiosemicarbazone derivatives. From the tested samples, the T2 derivative was the most thermally resistant (Tonset = 221.6 °C) in turn, the T11 derivative was the least thermally resistant (Tonset = 154.2 °C). Analysis of the melting peaks by c-DTA showed that the T15, and T17 derivatives melt at the highest temperatures (218.4 °C, 218.3 °C). On the other hand, the T4 derivative melts at the lowest temperature of 93.4 °C. The TGA measurements carried out showed that after one sample underwent one, and four stage decomposition. The remaining derivatives underwent two (13 samples), and three stage (4 samples) decomposition.
References
Hassan AA, Shawky AM, Shehatta HS. Chemistry and heterocyclization of thiosemicarbazones. J Heterocyclic Chem. 2012;49:21–37.
Bisceglie F, Tavone M, Mussi F, Azzoni S, Montalbano S, Franzoni S, Tarasconi P, Buschini A, Pelosi G. Effects of polar substituents on the biological activity of thiosemicarbazone metal complexes. J Inorg Biochem. 2018;179:60–70.
Yu P, Deng J, Cai J, Zhang Z, Zhang J, Hamid Khan M, Liang H, Yang F. Anticancer and biological properties of a Zn-2,6-diacetylpyridine bis(thiosemicarbazone) complex. Metallomics. 2019;11(8):1372–86.
Gaber A, Refat MS, Belal AAM, El-Deen IM, Hassan N, Zakaria R, Alhomrani M, Alamri AS, Alsanie WF, Saied M, E. New mononuclear and binuclear Cu(II), Co(II), Ni(II), and Zn(II) thiosemicarbazone complexes with potential biological activity: antimicrobial and molecular docking study. Molecules. 2021;26(8):2288.
Andres SA, Bajaj K, Vishnosky NS, Peterson MA, Mashuta MS, Buchanan RM, Bates PJ, Grapperhaus CA. Synthesis, characterization, and biological activity of hybrid thiosemicarbazone-alkylthiocarbamate metal complexes. Inorg Chem. 2020;59(7):4924–35.
Deng J, Yu P, Zhang Z, Wang J, Cai J, Wu N, Sun H, Liang H, Yang F. Designing anticancer copper(II) complexes by optimizing 2-pyridine-thiosemicarbazone ligands. Eur J Med Chem. 2018;158:442–52.
Bacher F, Enyedy EA, Nagy NV, Rockenbauer A, Bognár GM, Trondl R, Novak MS, Klapproth E, Kiss T, Arion VB. Copper(II) complexes with highly water-soluble l- and d-proline–thiosemicarbazone conjugates as potential inhibitors of topoisomerase Iiα. Inorg Chem. 2013;52:8895–908.
Khan SA, Asiri AM. Synthesis and spectroscopic studies of Ru(II) complexes of steroidal thiosemicarbazones by multi step reaction: as anti-bacterial agents. Steroids. 2017;124:23–8.
He Z, Qiao H, Yang F, Zhou W, Gong Y, Zhang X, Wang H, Zhao B, Ma L, Liu HM, Zhao W. Novel thiosemicarbazone derivatives containing indole fragment as potent and selective anticancer agent. Eur J Med Chem. 2019;184:111764.
Matsa R, Makam P, Kaushik M, Hoti SL, Kannan T. Thiosemicarbazone derivatives: design, synthesis and in vitro antimalarial activity studies. Eur J Pharm Sci. 2019;137:104986.
Matesanz AI, Souza P. Alpha-N-heterocyclic thiosemicarbazone derivatives as potential antitumor agents: a structure-activity relationships approach. Mini Rev Med Chem. 2009;2009:1389–96.
Jiang ZG, Lebowitz MS, Ghanbari HA. Neuroprotective activity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (PAN-811), a cancer therapeutic agent. CNS Drug Rev. 2006;12(1):77–90.
Singhal S, Arora S, Agarwal S, Sharma R, Singhal N. A review on potential biological activities of thiosemicarbazides. World J Pharm and Pharm Sci. 2013;2:4661–81.
Pham VH, Phan TPD, Phan DC, Vu BD. Synthesis and bioactivity of thiosemicarbazones containing adamantane skeletons. Molecules. 2020;25(2):324.
Hałdys K, Latajka R. Thiosemicarbazones with tyrosinase inhibitory activity. Medchemcomm. 2019;10(3):378–89.
Kaplancıklı ZA, Altıntop MD, Sever B, Cantürk Z, Özdemir A. Synthesis and in vitro evaluation of new thiosemicarbazone derivatives as potential antimicrobial agents. J Chem. 2016. https://doi.org/10.1155/2016/1692540.
Opletalová V, Doležel J. Thiosemicarbazones and their antimycobacterial effects. Ceska Slov Farm. 2013;62(2):78–83.
Pitucha M, Korga-Plewko A, Czylkowska A, Rogalewicz B, Drozd M, Iwan M, Kubik J, Humeniuk E, Adamczuk G, Karczmarzyk Z, Fornal E, Wysocki W, Bartnik P. Influence of complexation of thiosemicarbazone derivatives with Cu (II) Ions on their antitumor activity against melanoma cells. Int J Mol Sci. 2021;22(6):3104.
ICH. ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2 (R1): Geneva, Switzerland: ICH; 2005.
European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.). 10th ed. Strasbourg Cedex, France: EDQM; 2021.
Monajjemzadeh F, Ghaderi F. Thermal analysis methods in pharmaceutical quality control. J Mol Pharm Org Process Res. 2015;3:1–2.
Dołega A, Juszczynska-Gałazka E, Deptuch A, Jaworska-Goła T, Zielinski PM. Thermoanalytical studies of a cytotoxic derivative of carbamazepine: iminostilbene. Therm Anal Calorim. 2021;146:2151–60.
Ramos P, Broncel M. Influence of storage conditions on the stability of gum arabic and tragacanth. Molecules. 2022;27(5):1–16.
Rojek B, Wesołowski M. Compatibility study of theophyline with excipients using thermogravimetry supported by kinetic analysis. J Therm Anal Calorim. 2021;143:227–36.
Guo W, Li C, Du P, Wang Y, Zhao S, Wang J, Yang C. Thermal properties of drug polymorphs: a case study with felod pine form I and form IV. J Saudi Chem Soc. 2020;24:474–83.
Pirayavaraporn C, Rades T, Tucker IG. Determination of moisture content in relation to thermal behaviour and plasticization of Eudragit RLPO. Int J Pharm. 2012;422(1–2):68–74.
Ouerfelli N, Vrinceanu N, Mliki E, Homeida AM, Amin KA, Ogrodowczyk M, Alshehri FS, Ouerfelli N. Modeling of the irradiation efect on some physicochemical properties of metoprolol tartrate for safe medical uses. Sci Rep. 2020;10(67):1–12.
Clinical and Laboratory Standards Institute, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M07‒A11. Wayne, P. A., 2018.
CLSI M24, 3rd ed. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 3rd Edition. 38(21).
Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36(2):362–6.
Reck F, Alm R, Brassil P, Newman J, Dejonge B, Eyermann CJ, Breault G, Breen J, Comita-Prevoir J, Cronin M, Davis H, Ehmann D, Galullo V, Geng B, Grebe T, Morningstar M, Walker P, Hayter B, Fisher S. Novel N-linked aminopiperidine inhibitors of bacterial topoisomerase type II: broad-spectrum antibacterial agents with reduced hERG activity. J Med Chem. 2011;54(22):7834–47.
Biskup I, Golonka I, Gamian A, Sroka Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy Hig Med Dosw. 2013;67:958–63.
Wojtunik-Kulesza KA, Oniszczuk A, Oniszczuk T, Waksmundzka-Hajnos M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed Pharmacother. 2016;78:39–49.
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and oxidative defense. World Allergy Organ J. 2012;5:9–19.
Lawson MK, Valko M, Cronin MTD, Jomová K. Chelators in iron and copper toxicity. Curr Pharmacol Rep. 2016;2:271–80.
Sarker D, Hossen F, Zahan KE, Haque M, Zamir R, Asraf A. Synthesis, characterization, thermal analysis and antibacterial activity of Cu (II) and Ni(II) complexes with thiosemicarbazone derived from thiophene-2-aldehyde. J Mater Sci Res and Rev. 2020;5(2):15–25.
Akash MSH, Rehman K. Differential thermal analysis. In: Essentials of Pharmaceutical Analysis. Singapore: Springer; 2020.
Bartyzel A, Kaczor AA, Głuchowska H, Pitucha M, Wróbel TM, Matosiuk D. Thermal and spectroscopic studies of 2,3,5,-trisubstituted and 1,2,3,5-tetrassubstituted indoles as non-competitive antagonists of GluK1/GluK2 receptors. J Therm Anal Calorim. 2018;133:935–44.
Mirakami FS, Lang KL, Mendes C, Cruz AP, Carvalho Filho MAS, Silva MAS. Physico-chemical solid-state characterization of omeprazole sodium: thermal, spectroscopic and crystallinity studies. J Pharm Biomed Anal. 2009;49(1):72–80.
Author information
Authors and Affiliations
Contributions
PM—conceptualization, methodology, supervisor; PR—thermal analysis; KW—Kulesza research on antioxidant activity; JS—research on antimicrobial activity; AG, EA-K—research on anti-tuberculosis activity; DM—synthesis of compounds; KD—spectral analysis for the identification of compounds.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pitucha, M., Ramos, P., Wojtunik-Kulesza, K. et al. Thermal analysis, antimicrobial and antioxidant studies of thiosemicarbazone derivatives. J Therm Anal Calorim 148, 4223–4234 (2023). https://doi.org/10.1007/s10973-023-12029-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12029-z