Abstract
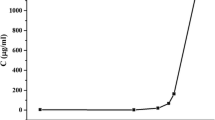
Solid dispersions (SD) are used as a technological strategy to increase the aqueous dissolution rate of poorly soluble active pharmaceutical ingredients, such as hydrochlorothiazide (HCTZ), an antihypertensive used frequently in medical clinics. The aim of this study was to characterize solid dispersions of HCTZ obtained with different processing adjuvants, using the DSC, TG, XRPD, FTIR and SEM techniques, and to evaluate the influence of carriers used in biopharmaceutical performance by analyzing dissolution efficiency. The SDs were obtained using the solvent method, and spray drying was used as the drying technique. The carriers used PEG 1500, sodium lauryl sulfate and PVP K30. The calorimetric analysis and XRPD showed amorphous behavior to SDs that used hydrophilic polymer as a carrier, and thermogravimetric analysis showed maintaining thermal stability of the HCTZ for most dispersions. FTIR detected intermolecular interactions of hydrogen bonds, while SEM showed the formation of microparticles with a tendency to sphericity. Acquired morphology associated with amorphization contributed to the increase in dissolution efficiency of dispersions, being that this SD (HCTZ/PVP K30) showed the best increase in dissolution. We therefore concluded that the analytical techniques used were of fundamental importance to the characterization of pharmaceutical products developed as to their physicochemical properties and the prescience of the oral bioavailability of HCTZ.






Similar content being viewed by others
References
Alves LDS, Lyra MAM, Rolim LA, Presmich GMA, Rolim-Neto PJ. Avanços, propriedades e aplicações de dispersões sólidas no desenvolvimento de formas farmacêuticas sólidas. Rev Ciênc Farm Básica Apl. 2012;33:17–25.
Truong DH, Tran TH, Ramasamy T, Choi JY, Choi H-G, Yong CS, Kim JO. Preparation and characterization of solid dispersion using a novel amphiphilic copolymer to enhance dissolution and oral bioavailability of sorafenib. Powder Technol. 2015;283:260–5.
Bikiaris D, Papageorgiou GZ, Stergiou A, Pavlidou E, Karavas E, Kanaze F, Georgarakis M. Physicochemical studies on solid dispersions of poorly water-soluble drugs evaluation of capabilities and limitations of thermal analysis techniques. Thermochim Acta. 2005;439:58–67.
Vo CL-N, Park C, Lee B-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85:799–813.
Veronez IP, Daniel JSP, Júnior CEC, Garcia JS, Trevisan MG. Development, characterization, and stability studies of ethinyl estradiol solid dispersion. J Therm Anal Calorim. 2015;120:573–81.
Janssens S, de Armas HN, D’Autry W, Van Schepdael A, Van den Mooter G. Characterization of ternary solid dispersions of Itraconazole in polyethylene glycol 6000/polyvidone-vinylacetate 64 blends. Eur J Pharm Biopharm. 2008;69:1114–20.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. Int Sch Res Netw. 2012;. doi:10.5402/2012/195727.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvente evaporation and supercritical methods. Int J Pharm. 2004;272:1–10.
Mogal SA, Gurjar PN, Yamgar DS, Kamod AC. Solid dispersion technique for improving solubility of some poorly soluble drugs. Der Pharm Lett. 2012;4:1574–86.
Kaur P, Singh SK, Garg V, Gulati M, Vaidya Y. Optimization of spray drying process for formulation of solid dispersion containing polypeptide-k powder through quality by design approach. Powder Technol. 2015;284:1–11.
Chauhan B, Shimpi S, Paradkar A. Preparation and characterization of etoricoxib solid dispersions using lipid carriers by spray drying technique. AAPS Pharm Sci Tech. 2005;6:E405–12.
British pharmacopoeia. The stationary office: pharmacopeia Commission British. Pharmabooks Publisher. 2015; ISBN 10 0113229879.
Brunton LL, Chabner BA, Knollmann BC. Goodman and Gilman: as bases Farmacológicas da Terapêutica. 12th ed. Rio de Janeiro: McGraw-Hill; 2012.
Corveleyn S, Remon JP. Bioavailability of hydrochlorothiazide: conventional versus freeze-dried tablets. Int J Pharm. 1998;173:149–55.
Chadha R, Bhandari S, Khullar S, Mandal SK, Jain DV. Characterization and evaluation of multi-component crystals of hydrochlorothiazide. Pharm Res. 2014;31:2479–89.
El-Gizawy SA, Osman MA, Arafa MF, El Maghraby GM. Aerosil as a novel co-crystal co-former for improving the dissolution rate of hydrochlorothiazide. Int J Pharm. 2015;478:773–8.
Khan A, Iqbal Z, Shah Y, Ahmad L, Ullah Z, Ullah A. Enhancement of dissolution rate of class II drugs (hydrochlorothiazide); a comparative study of the two novel approaches; solid dispersion and liqui-solid techniques. Saudi Pharm J. 2015;23:650–7.
Fengming L. Candesartan hydrochlorothiazide dispersible tablets and the preparing method thereof. CN101062038A; 2007.
Roller N. A Losartan potassium hydrochlorothiazide pharmaceutical composition liposome solid preparation. CN101797230A; 2010.
Minggui Y. Lisinopril and hydrochlorothiazide pharmaceutical composition liposome solid preparation. CN102166208B; 2011.
Chongkai G; Huiqiu H; Ning L; Jie J. Osmotic pump controlled release tablet of losartan potassium and hydrochlorothiazide solid dispersion or inclusion compound. CN103006566 A; 2013.
Górrniak A, Gajda M, Pluta J, Czapor-Irzabek H, Karolewicz B. Thermal, spectroscopic and dissolution studies of lovastatin solid dispersions with acetylsalicylic acid. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-016-5279-z.
Khan KA, Rhodes CT. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9.
Oliveira MA, Yoshida MI, Silva DCGM. Quality evaluation of pharmaceutical formulations containing hydrochlorothiazide. Molecules. 2014;19:16824–36.
Brazilian pharmacopoeia. Farmacopéia Brasileira. 5th ed. Brasília: Agência Nacional de Vigilância Sanitária; 2010. p. 546.
Mashru RC, Sutariya VB, Sankalia MG, Yagnakumar P. Characterization of solid dispersions of rofecoxib using differential scanning calorimeter. J Therm Anal Calorim. 2005;82:167–70.
Yu M, Sun L, Li W, Lan Z, Li B, Tan L, Li M, Yang X. Investigation of structure and dissolution properties of a solid dispersion of lansoprazole in polyvinylpyrrolidone. J Mol Struct. 2011;1005:70–7.
Shi C, Tong Q, Fang J, Wang C, Wu J, Wang W. Preparation, characterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcholine. Eur J Pharm Sci. 2015;74:11–7.
LaFountaine JS, Prasad LK, Brough C, Miller DA, McGinity JW, Williams RO III. Thermal processing of PVP- and HPMC-based amorphous solid dispersions. AAPS Pharm Sci Tech. 2016;17:120–32.
Nath MR, Saria M. Synthesis: isolation and characterization of hydrochlorothiazide dimer impurity. Int J Pharm Pharm Sci. 2013;5:867–71.
Padma PS, Rajendran NN, Lakshmi PK, Umadevi SK, Vaijayanthy V, Kausalya J, Ravichandran V. A novel captopril hydrochlorothiazide solid dispersion. Int J Pharm Pharm Sci. 2010;2:30–2.
Fousteris E, Tarantili PA, Karavas E, Bikiaris D. Poly(vinyl pyrrolidone)–poloxamer-188 solid dispersions prepared by hot melt extrusion: thermal properties and release behavior. J Therm Anal Calorim. 2013;113:1037–47.
Trivedi RV, Admane PS, Taksande JB, Mahore JG, Umekar MJ. Solubility enhancement studies of hydrochlorothiazide by preparing solid dispersions using losartan potassium and urea by different methods. Der Pharm Lett. 2011;3:8–17.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Ansel HC, Popovich NG, Allen LV. Formas Farmacêuticas and Sistemas de Liberação de Fármacos. 8th ed. São Paulo: Artmed; 2007.
Vieira ACQM, Marques GS, Melo CM, Silva KER, Rolim LA, Lima MCA, Galdino SL, Pitta IR, Rolim-Neto PJ. Physical–chemical characterization of new anti-inflammatory agent (LPSF/GQ-130) and evaluation of its thermal compatibility with pharmaceutical excipients. J Therm Anal Calorim. 2014;115:2339–49.
Storpirtis S, Gonçalves JE, Chiann C, Gai MN. Biofarmacotécnica. 2ª ed. Rio de Janeiro: Editora Guanabara Koogan; 2011.
United States Pharmacopea and National Formulary USP 29-NF 24. Pharmacopeial Convention, Rockville; 2010.
Mahle F, Goelzer F, Adriano J, Felippe M, Vier N, Carli RBG, Rosa T, Couto AG, Lucinda-Silva RM. Avaliação do perfil de dissolução de comprimidos de hidroclorotiazida comercializados no Brasil. Rev Ciênc Farm Básica Apl. 2007;28:265–71.
Acknowledgements
We thank Coordination of Improvement of Higher Level Personnel (CAPES) for funding this work and to Laboratory of Evaluation and Development of Northeastern Biomaterials (CERTBIO) of the Federal University of Campina Grande and to Materials Engineering Laboratory of the Federal University of Paraíba by analysis provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, C.M.P., dos Santos, J.A.B., do Nascimento, A.L. et al. Thermal analysis study of solid dispersions hydrochlorothiazide. J Therm Anal Calorim 131, 681–689 (2018). https://doi.org/10.1007/s10973-017-6091-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6091-0