Abstract
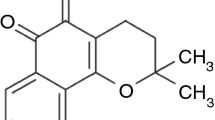
LPSF/GQ-130 is a drug candidate, according to reports about its significant anti-inflammatory activity and non-toxicity demonstrated in an acute preclinical study. Despite this, knowledge of its physical–chemical properties is insufficient for the development of medicines. Thus, this work aimed to characterize the raw material at its molecular, particle, and agglomerate level as well as evaluate its thermal compatibility to pharmaceutical excipients. Through spectrometric techniques the molecular structure of the substance was confirmed. For thermal analysis its melting (171.3–176.5 °C) and degradation (238.3–297.4 °C) ranges, besides its purity (99.37 %), were determined. The kinetic non-isothermal degradation supplied the order of thermal reaction (0), the activation energy (96.14 kJ mol−1) and the frequency factor (3.130 × 10−7 min−1). The diffraction of X-rays presented well defined signs in the angles 5.5°, 16.3°, and 44.18° 2θ, suggesting crystalline structure. Scanning electronic microscopy exhibited needle morphology. LPSF/GQ-130 presented Type-III isotherm adsorption/desorption, with a superficial area of 81.3529 m2 g−1 and water content calculated at 1 % using the Karl Fisher method. Laser granulometry calculated its granulometry between 11.65 and 13.10 μm, thus it was characterized as a very fine powder. The prototype was classified as insoluble in water (<0.0187 μg mL−1) and soluble in acetone and acetonitrile, and exhibits instability in basic pH (100 %) and oxidative conditions (30–70 %). In thermal compatibility the excipients PVP K-30, Compritol® 888 ATO, and MYRJ® 59 seem to exercise a protective thermal activity for the prototype.











Similar content being viewed by others
References
Liesen AP, Aquino TM, Góes AJS, Lima JG, Faria AR, Alves AJ. Métodos de obtenção, reatividade e importância biológica de 4-tiazolidinonas. Quím Nova. 2008;31:369–76.
Buckingham RE. Thiazolidinediones: Pleiotropic drugs with potent anti-inflammatory properties for tissue protection. Hepatol Res. 2005. doi:10.1016/j.hepres.2005.09.027.
Barnett AH. Redefining the role of thiazolidinediones in the management of type 2 diabetes. Vasc Health Risk Manag. 2009;5:141–51.
Chen SD, Wu HY, Yang DI, Lee SY, Shaw FZ, Lin TK, Liou CW, Chuang YC. Effects of rosiglitazone on global ischemia-induced hippocampal injury and expression of mitochondrial uncoupling protein 2. Biochem Biophys Res Commun. 2006;351:198–203.
Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisomes proliferators-activated receptor-γ agonist rosiglitazone. J Neurochem. 2006;97:435–48.
Pereira MP, Hurtado O, Cárdenas A, Boscá L, Castillo J, Dávalos A, Vivancos J, Serena J, Lorenzo P, Lizasoian I, Moro MA. Rosiglitazone and 15-deoxy-∆12,14-prostaglandin J2cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J Cereb Blood Flow Metab. 2006;26:218–29.
Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor γ in microglia/macrophages. Ann neurol. 2007;61:352–62.
Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology LXI peroxisome proliferator-activated receptors. Pharmacology. 2006;58:726–41.
Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharm Ther. 2006;110:371–85.
Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, Chung HY. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res Rev. 2008;7:126–36.
Souza TRCL, Marques GS, Vieira ACQM, Freitas JCR. State of the art of anti-inflammatory drugs. Pharmacotherapy. 1st ed. Croácia: InTech; 2012.
White AT, Murphy AN. Administration of thiazolidinediones for neuroprotection in ischemic stroke: a pre-clinical systematic review. J Neurochem. 2010. doi:10.1111/j.1471-4159.2010.06999.
Santos LC, Uchôa FT, Moura RO, Lima MCA, Galdino SL, Pitta IR, Baerbe J. Synthesis and anti-inflammatory activity of new thiazolidine-2,4-diones, 4-thioxothiazolidinoes and 2-thioxoimidazolidinones. Heterocycl Commun. 2005;11:121–8.
Rodrigues OP, Cardoso TFM, Silva MAF, Matos JR. Aplicação de Técnicas Termoanalíticas na Caracterização, Determinação da Pureza e Cinética de Degradação da Zidovudina (AZT). Acta farm Bonaer. 2005;24:383–7.
Gomes P, Sipel J, Jablonski A, Steppe M. Determination of rosiglitazone in coated tablets by MEKC and HPLC methods. J Pharmaceut Biomed. 2004;36:909–13.
Veranasi VSKK, Veeraraghavan S, Potharaju S, Satheeshmanikandan TRS, Raghavan R, Swaroop KVVS. Validated high performance liquid chromatographic method for simultaneous determination of rosiglitazone, cilostazol, and 3,4-dehydro-cilostazol in rat plasma and its application to pharmacokinetics. Arzneimittelforschung. 2008. doi:10.1055/s-0031-1296509.
Ozawa TA. New method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa TA. Thermal analysis: review and prospect. Thermochim Acta. 2000;355:35–42.
Rodante F, Catalani G, Vecchio S. Kinetic analysis of single or multi-step decomposition processes-limits introduced by statistical analysis. J Therm Anal Calorim. 2002;68:689–713.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behaviour, compatibility study and decompositions kinetics of glimepiride under isothermal and nonisothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
FARMACOPÉIA brasileira. 5.ed. Brasília: Anvisa, 2010. Part. 1, p. 125.
Chowdary KPR, Chandra DU, Mahesh N, Reddy TM, Gopaiah KV. Enhancement of dissolution rate and formulation development of pioglitazone – A BCS class II drug. J Pharm Res. 2011;4:3862–3.
Mishra SR, Ellaiah P, Jena PK, Nayak BS, Mishra G. An approach for enhancement of dissolution rate of pioglitazone HCl by solid dispersion. J Pharm Sci. 2011;2:2681–5.
Ahmed S, Ahmad Z, Baraskar N, Ahmed U, Siddiqui AR. Dissolution rate enhancement of pioglitazone hydrochloride by surface ternary solid dispersion. J Pharm Res. 2011;4:3606–8.
Carvalho JP, Santos AS, Sá AS, Teixeira CS, Nogueira MS. Estabilidade de medicamentos no âmbito da farmacovigilância. Fármacos e Medicamentos. 2008;102:22–7.
Oliveira MA, Yoshida MI, Gomes CL. Análise térmica aplicada a fármacos e formulações farmacêuticas na indústria farmacêutica. Quím Nova. 2011;34:1–7.
Araújo AAS, Bezerra MS, Storpirtis S, Matos JR. Determination of the melting temperature, heat of fusion, and purity analysis of different samples of zidovudine (AZT) using DSC. Braz J Pharm Sci. 2010;46:37–43.
Brown ME. Introduction to thermal analysis: techniques and applications. 2nd ed. Norwell: Kluer Academic Publishers; 2001.
Tiţa B, Marian E, Tiţa D, Vlase G, Doca N, Vlase T. Comparative kinetic study of decomposition of some diazepine derivatives under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2008;94:447–52.
Soares-Sobrinho JL, Soares MFR, Lopes PQ, Correia LP, Souza FS, Macêdo RO, Rolim-Neto PJ. A preformulation study of a new medicine for chagas disease treatment: physicochemical characterization, thermal stability, and compatibility of benznidazole. AAPS Pharm Sci Tech. 2010;11:1391–6.
Sovizi MR. Investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102:285–9.
Cheng Y, Huang Y, Alexander K, Dollimore D. A thermal analysis study of methyl salicylate. Thermochim Acta. 2001;367:23–8.
Rodante F, Vecchio S, Catalani G, Tomassetti M. Application of TA and kinetic study to compatibility and stability problems in some commercial drugs. Remarks on statistical data. J Therm Anal Calorim. 2001;66:155–78.
Felix FS, Cides LCS, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of Salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95:181–214.
Giordano F, Rossi A, Pasquali I, Bettini R, Frigo E, Gazzaniga A, Sangalli ME, Mileo V, Catinela SJ. Thermal degradation and melting point determination of diclofenac. Therm Anal Calorim. 2003;73:509–18.
Lachman L, Lieberman HA, Kanig JL. Teoria e prática na indústria farmacêutica. Lisboa: Fundação Calouste Gulbenkian; 2001.
International Organization for Standardization. ISO 13.320-1:1999: Particle size analysis laser diffraction methods—Part 1: general principles. Geneva, 1999.
Byung-man K, Lee JE, Jang-hyuk A, Tae-hong J. Laser diffraction particle sizing by wet dispersion method for spray-dried infant formula. J Food Eng. 2008;92:324–30.
Castellanos A, Valverde JM, Pérez AT, Ramos A, Watson PK. Flow regimes in fine cohesive powders. Phys Rev Lett. 1999;82:6–9.
Navaneethan CV, Missaghi S, Fassihi R. Application of powder rheometer to determine powder flow properties and lubrication efficiency of pharmaceutical particulate systems. AAPS Pharm Sci Tech. 2005;6:E398–404.
Sarraguça MC, Cruz AV, Soares SO, Amaral HR, Costa PC, Lopes JA. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J Pharmaceut Biomed. 2010;52:484–92.
Torrado S, Torrado S. Characterization of physical state of mannitol after freezing-drying: effect of Acetylsalicylic acid as a second crystalline cosolute. Chem Pharm Bull. 2002;50:567–70.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Del Rev. 2004;56:335–47.
Nery CGC, Arlete M, Pianetti GA, Vianna-Soares CD. Caracterização do fármaco hipoglicemiante glibenclamida. Rev Bras Cienc Farm. 2008;44:61–73.
Teixeira VG, Coutinho FMB, Gomes AS. Principais métodos de caracterização da porosidade de resinas à base de divinilbenzeno. Quím Nova. 2001;24:808–18.
Saifee M, Inamdar M, Dhamecha DL, Rathi AA. Drug polymorphism: a review. Int J Health Res. 2009;2:289–306.
Yu D-G, Yang J-M, Branford-White C, Lu P, Zhang L, Zhu L-M. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int J pharm. 2010. doi:10.1016/j.ijpharm.
Darlene L, Alves S, Andreza M, Lyra MD, Rolim LA, Maria G, Presmich A. Avanços, propriedades e aplicações de dispersões sólidas no desenvolvimento de formas farmacêuticas sólidas. Rev Ciênc Farm Básica Apl. 2012;33:17–25.
Venkatesh P, Harisudhan T, Choudhury H, Mullangi R, Nuggehally RS. Simultaneous estimation of six anti-diabetic drugs— glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomed Chromatogr. 2006;20:1043–8.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91:323–8.
Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS Pharm. Sci. Tech. 2006;7:E87–92.
Tiţa B, Fuliaş A, Szabadai Z, Rusu G, Bandur G, Tiţa D. Compatibility study between ibuprofen and excipients in their physical mixtures. J Therm Anal Calorim. 2010;105:517–27.
Acknowledgements
Centro de Tecnologias Estratégicas do Nordeste (CETENE), Fundação de Ciência e Tecnologia do Estado de Pernambuco, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Medeiros Vieira, A.C.Q., Marques, G.S., de Melo, C.M. et al. Physical–chemical characterization of new anti-inflammatory agent (LPSF/GQ-130) and evaluation of its thermal compatibility with pharmaceutical excipients. J Therm Anal Calorim 115, 2339–2349 (2014). https://doi.org/10.1007/s10973-013-3358-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3358-y