Abstract
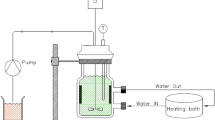
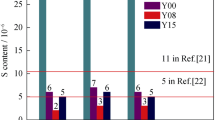
Owing to its capability of high-temperature operation, a salt waste treatment process in a pyrochemical process which uses molten salt as an electrolyte for the recycling of spent nuclear fuel adopted melt-crystallization method to purify a molten salt waste generated during the processes. However, because of its high operating temperature, there is a corrosion problem in the crystallizer made of Inconel 625 alloy, resulting in the contamination of crystallized salt on the crystallization plates. To solve the corrosion problem stemming from the molten salt waste under high operation temperature, a reactive-crystallization method was suggested in this study, in which the corrosion products are precipitated by the addition of lithium carbonate as a precipitation agent. To determine the effectiveness of the process, the concentration of corrosion products were analyzed for each stage of the process with respect to the values obtained using the previous melt-crystallization method. In addition, the nuclide separation efficiencies of group I/II and rare-earth nuclides were investigated using the reactive-crystallization method. Through the study, it is found that the reactive-crystallization method is effective in terms of removal of corrosion products, thus resulting in high nuclide separation efficiency.







Similar content being viewed by others
References
Park GI, Jeon MK, Choi JH, Lee KR, Han SY, Kim IT, Cho YZ, Park HS (2019) Recent progress in waste treatment technology for pyroprocessing at KAERI. J Nucl Fuel Cycle Waste Technol 17:279–298
Lee HS, Hur JM, Kim JG, Ahn DH, Cho YZ, Paek SW (2011) Korean Pyrochemical Process R&D activities. Energy Procedia 7:391–395
Lee HS, Park GI, Kang KH, Hur JM, Kim JG, Ahn DH, Cho YZ, Kim EH (2011) Pyroprocessing technology development at KAERI. Nucl Eng Technol 43:317–328
Lee HS, Park GI, Lee JW, Kang KH, Hur JM, Kim JG, Paek SW, Kim IT, Cho IJ (2013) Current status of pyroprocessing development at KAERI. Sci Technol Nucl Install 2013:1–11
Park BH, Park SB, Jeong SM, Seo SS, Park SW (2006) Electrolytic reduction of spent oxide fuel in a molten LiCl–Li2O system. J Radioanal Nucl Ch 270:575–583
Choi EY, Won CY, Kang DS, Kim SW, Cha JS, Lee SJ, Park WS, Im HS, Hur JM (2015) Production of uranium metal via electrolytic reduction of uranium oxide in molten LiCl and salt distillation. J Radioanal Nucl Ch 304:535–546
Kim SW, Jeon MK, Choi EY (2019) Electrolytic behavior of SrCl2 and BaCl2 in LiCl molten salt during oxide reduction in pyroprocessing. J Radioanal Nucl Ch 321:361–365
Cho YZ, Ahn BG, Eun HC, Jung JS, Lee HS (2011) Melt crystallization process treatment of LiCl salt waste generated from electrolytic reduction process of spent oxide fuel. Energy Procedia 7:525–528
Cho YZ, Park GH, Lee HS, Kim IT, Han DS (2010) Concentration of cesium and strontium elements involved in a LiCl waste salt by a melt crystallization process. Nucl Technol 171:325–334
Choi JH, Cho YZ, Lee TK, Eun HC, Kim JH, Kim IT, Park GI, Kang JK (2013) Inclusion behavior of Cs, Sr, and Ba impurities in LiCl crystal formed by layer-melt crystallization: combined first-principles calculation and experimental study. J Cryst Growth 371:84–89
Choi JH, Lee TK, Lee KR, Han SY, Cho YZ, Kim NY, Jang SA, Park HS, Hur JM (2018) Melt-crystallization monitoring system for the purification of 10 kg-scale LiCl salt waste. Nucl Eng Des 326:1–6
Hofmeister M, Klein L, Miran H, Rettig R, Virtanen S, Singer RF (2015) Corrosion behaviour of stainless steels and a single crystal superalloy in a ternary LiCl–KCl–CsCl molten salt. Corros Sci 90:46–53
Roine A (2018) HSC Chemistry® [Software]. Outotec, Pori
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2017M2A8A5015082).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, JH., Lee, KR., Kang, HW. et al. Reactive-crystallization method for purification of LiCl salt waste. J Radioanal Nucl Chem 325, 485–492 (2020). https://doi.org/10.1007/s10967-020-07235-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07235-0