Abstract
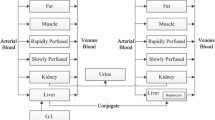
Selecting the appropriate pharmacokinetic (PK) model given the available data is investigated for perfluorooctanoic acid (PFOA), which has been widely analyzed with an empirical, one-compartment model. This research examined the results of experiments [Kemper R. A., DuPont Haskell Laboratories, USEPA Administrative Record AR-226.1499 (2003)] that administered single oral or iv doses of PFOA to adult male and female rats. PFOA concentration was observed over time; in plasma for some animals and in fecal and urinary excretion for others. There were four rats per dose group, for a total of 36 males and 36 females. Assuming that the PK parameters for each individual within a gender were drawn from the same, biologically varying population, plasma and excretion data were jointly analyzed using a hierarchical framework to separate uncertainty due to measurement error from actual biological variability. Bayesian analysis using Markov Chain Monte Carlo (MCMC) provides tools to perform such an analysis as well as quantitative diagnostics to evaluate and discriminate between models. Starting from a one-compartment PK model with separate clearances to urine and feces, the model was incrementally expanded using Bayesian measures to assess if the expansion was supported by the data. PFOA excretion is sexually dimorphic in rats; male rats have bi-phasic elimination that is roughly 40 times slower than that of the females, which appear to have a single elimination phase. The male and female data were analyzed separately, keeping only the parameters describing the measurement process in common. For male rats, including excretion data initially decreased certainty in the one-compartment parameter estimates compared to an analysis using plasma data only. Allowing a third, unspecified clearance improved agreement and increased certainty when all the data was used, however a significant amount of eliminated PFOA was estimated to be missing from the excretion data. Adding an additional PK compartment reduced the unaccounted-for elimination to amounts comparable to the cage wash. For both sexes, an MCMC estimate of the appropriateness of a model for a given data type, the Deviance Information Criterion, indicated that this two-compartment model was better suited to describing PFOA PK. The median estimate was 142.1 ± 37.6 ml/kg for the volume of the primary compartment and 1.24 ± 1.1 ml/kg/h for the clearances of male rats and 166.4 ± 46.8 ml/kg and 30.3 ± 13.2 ml/kg/h, respectively for female rats. The estimates for the second compartment differed greatly with gender—volume 311.8 ± 453.9 ml/kg with clearance 3.2 ± 6.2 for males and 1400 ± 2507.5 ml/kg and 4.3 ± 2.2 ml/kg/h for females. The median estimated clearance was 12 ± 6% to feces and 85 ± 7% to urine for male rats and 8 ± 6% and 77 ± 9% for female rats. We conclude that the available data may support more models for PFOA PK beyond two-compartments and that the methods employed here will be generally useful for more complicated, including PBPK, models.










Similar content being viewed by others
References
Andersen ME, Clewell HJ, Frederick CB (1995) Applying simulation modeling to problems in toxicology and risk assessment—a short perspective. Toxicol Appl Pharmacol 133:181–187
Gelman A, Bois FY, Jiang J (1996) Physiological pharmacokinetic analysis using population modeling and informative prior distributions. J Am Stat Assoc 91:1400–1412
Parham FM, Matthews HB, Portier CJ (2002) A physiologically based pharmacokinetic model of p,p′-dichlorodiphenylsulfone. Toxicol Appl Pharmacol 181:153–163
Keys DA, Wallace DG, Kepler TB, Conolly RB (1999) Quantitative evaluation of alternative mechanisms of blood and testes disposition of di(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in rats. Toxicol Sci 49:172–185
Collins AS, Sumner SCJ, Borghoff SJ, Medinsky MA (1999) A physiological model for tert-amyl methyl ether and tert-amyl alcohol: hypothesis testing of model structures. Toxicol Sci 49:15–28
Cobelli C, DiStefano JJ (1980) Parameter and structural identifiability concepts and ambiguities: a critical review and analysis. Am J Physiol Regul, Integr Comp Physiol 239:7–24
Bernillon P, Bois FY (2000) Statistical issues in toxicokinetic modeling: a Bayesian perspective. Environ Health Perspect 108:883–893
Mezzetti M, Ibrahim JG, Bois FY, Ryan LM, Ngo L, Smith TJ (2003) A Bayesian compartmental model for the evaluation of 1,3-butadiene metabolism. J R Stat Soc Ser C-Appl Stat 52:291–305
Gueorguieva I, Aarons L, Rowland M (2006) Diazepam pharamacokinetics from preclinical to phase I using a Bayesian population physiologically based pharmacokinetic model with informative prior distributions in Winbugs. J Pharmacokinet Pharmacodyn 33:571–594
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305
Andersen ME, Clewell HJ, Tan Y-M, Butenhoff JL, Olsen GW (2006) Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys-probing the determinants of long plasma half-lives. Toxicology 227:156–164
Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115:1596–1602
Washburn ST, Bingman TS, Braithwaite SK, Buck RC, Buxton LW, Clewell HJ, Haroun LA, Kester JE, Rickard RW, Shipp AM (2005) Exposure assessment and risk characterization for perfluorooctanoate in selected consumer articles. Environ Sci Technol 39:3904–3910
Heuvel JPV, Kuslikis BI, Rafelghem MJV, Peterson RE (1991) Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol 6:83–92
Kudo N, Katakura M, Sato Y, Kawashima Y (2002) Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemico-Biol Interact 139:301–316
Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y (2007) Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J Health Sci 53:77–83
Nakagawa H et al. (2007) Roles of organic anion transporters in the renal excretion of perfluoroctanoic acid. Basic Clin Pharmacol Toxicol 103:1–8
Loveless SE, Finlay C, Everds NE, Frame SR, Gillies PJ, O’Connor JC, Powley CR, Kennedy GL (2006) Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (apfo). Toxicology 220:203–217
Kemper RA (2003) Perfluorooctanoic acid: toxicokinetics in the rat, DuPont Haskell Laboratories, Laboratory Project ID: DuPont-7473. USEPA Administrative Record AR-226.1499
Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, Zobel LR (2004) Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol 39:363–380
Portier CJ, Lyles CM (1996) Practicing safe modeling: GLP for biologically based mechanistic models. Environ Health Perspect 104:806
Sheiner LB, Beal SL (1980) Evaluation of methods for estimating population pharmacokinetic parameters. I. Michaelis–Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Pharmacodyn 8:553–571
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—a Bayesian modeling framework: concepts, structure and extensibility. Stat Comput 10:325–337
Lunn DJ, Best NG, Thomas A, Wakefield J, Spiegelhalter D (2002) Bayesian analysis of population PK/PD models: general concepts and software. J Pharmacokinet Pharmacodyn 29:271–307
Ihaka R, Gentleman R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Sturtz S, Ligges U, Gelman A (2005) R2WinBUGS: a package for running WinBUGS from R. J Stat Software, 12
Lunn DJ (2003) WinBUGS development interface (WBDev). ISBA Bull 10:10–11
Oberon Microsystems Inc (2001) Component pascal language report
Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Bayesian data analysis, 2nd edn. Chapman and Hall/CRC
Gelman A (2006) Prior distributions for variance parameters in hierarchical models. Bayesian Anal 1:515–533
Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR (2005) How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Stat Med 25:2401–2428
Rocke DM, Lorenzato S (1995) A two-component model for measurment error in analytic chemistry. Technometrics 37:176–184
Bois FY, Gelman A, Jiang J, Maszle D, Zeise L, Alexeef G (1996) Population toxicokinetics of tetrachloroethylene. Arch Toxicol 70:347–355
Chiu WA, Bois FY (2006) Revisiting the population toxicokinetics of tertracholoroethylene. Arch Toxicol 80:382–385
Best NG, Cowles MK, Vines K (1995) CODA: convergence diagnosis and output analysis for Gibbs sampling output
R Development Core Team (2008) R: a language and environment for statistical computing, ISBN 3-900051-07-0, URL http://www.R-project.org
Gelman A, Rubin DB (1992) Inferences from iterative simulation using multiple sequences. Stat Sci 7:457–472
Raftery AE, Lewis SM (1992) Comment: one long run with diagnostics: implementation strategies for Markov Chain Monte Carlo. Stat Sci 7:493–497
Dodds MG, Vicini P (2004) Assessing convergence of Markov Chain Monte Carlo simulations in hierarchical Bayesian models for populations pharmacokinetics. Ann Biomed Eng 32:1300–1313
Spiegelhalter DJ, Best NG, Carlin BP (2002) Bayesian measures of model complexity and fit. J R Stat Soc B 64:583–639
Spiegelhalter DJ, Best NG, Carlin BP (1998) Bayesian deviance, the effective number of parameters, and the comparison of arbitrarily complex models. Technical Report, Medical Research Council Biostatistics Unit, Cambridge, UK
Barton HA et al. (2007) Characterizing uncertainty and variability in physiologically based pharmacokinetic models: state of the science and needs for research and implementation. Toxicol Sci 99:395–402
Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A (2005) Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 99:253–261
Kudo N, Sakai A, Mitsumoto A, Hibino Y, Tsuda T, Kawashima Y (2007) Tissue distribution and hepatic subcellular distribution of perfluorooctanoic acid at low dose are different from those at high dose in rats. Biol Pharm Bull 30:1535–1540
Stone CJ, Hansen M, Kooperberg C, Truong YK (1997) The use of polynomial splines and their tensor products in extended linear modeling (with discussion). Ann Stat 25:1371–1470
Acknowledgments and Disclaimer
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. Interagency Agreement RW-75-92207501 with the National Toxicology Program at the National Institute for Environmental Health Science was a partial source of funding. This research has been subjected to Agency review and approved for publication.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wambaugh, J.F., Barton, H.A. & Setzer, R.W. Comparing models for perfluorooctanoic acid pharmacokinetics using Bayesian analysis. J Pharmacokinet Pharmacodyn 35, 683–712 (2008). https://doi.org/10.1007/s10928-008-9108-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-008-9108-2