Abstract
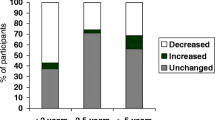
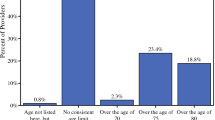
The purpose of this study was to evaluate the utility of a breast cancer risk assessment (BCRA) at the time of screening mammogram. Women whose BCRA indicated a high risk for cancer received a letter with instructions for breast health care and genetic counseling if appropriate. After 6 months this group received surveys to evaluate their risk perception and their recall of, and compliance with, recommendations. We also explored the impact of other variables such as a recommendation for genetic counseling and physician communication with the women. After the BCRA, the majority of high risk women reported no change in their perceived risk of cancer. A woman’s perceived risk of cancer after a BCRA was significantly associated with her recall of recommendations for breast health care, but not with compliance. A recommendation for genetic counseling was not significantly related to women’s perceived risk of cancer after the BCRA. Ten percent of women who should have obtained genetic counseling actually completed an appointment. Women who discussed their BCRA results with their physicians were more compliant with a six month breast exam with a doctor (53% vs 17%, p = 0.018). Overall, women felt that the BCRA was helpful and did not cause undue stress or anxiety. Although the cohort’s compliance with recommendations was suboptimal, physicians’ interactions with their patients may have a positive influence on their compliance.

Similar content being viewed by others
References
Aagaard-Tillery, K., Sibai, B., Spong, C. Y., Momirova, V., Wendel, G., Wenstrom, K., et al. (2006). Sample bias among women with retained DNA samples for future genetic studies. Obstetrics and Gynecology, 108(5), 1115–1120. doi:10.1097/01.AOG.0000241536.19539.14.
Calvocoressi, L., Kasl, S. V., Lee, C. H., Stolar, M., Claus, E. B., & Jones, B. A. (2004). A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and white women ages 40 to 79 years. Cancer Epidemiology, Biomarkers & Prevention, 13(12), 2096–2105.
Espeland, M. A., Dotson, K., Jaramillo, S. A., Kahn, S. E., Harrison, B., Montez, M., et al. Look AHEAD Research Group. (2006). Consent for genetics studies among clinical trial participants: findings from action for health in diabetes (look AHEAD). Clinical Trials (London, England), 3(5), 443–456. doi:10.1177/1740774506070727.
Evans, D. G. R., & Howell, A. (2007). Breast cancer risk-assessment models. Breast Cancer Research: BCR, 9(5), 213. doi:10.1186/bcr1750.
Evans, D. G. R., Ingham, S., Dawe, S., Roberts, L., Lalloo, F., Brentnall, A. R., et al. (2014). Breast cancer risk assessment in 8,824 women attending a family history evaluation and screening programme. Familial Cancer, 13(2), 189–196.
Ford, B. M., Evans, J. S., Stoffel, E. M., Balmaña, J., Regan, M. M., & Syngal, S. (2006). Factors associated with enrollment in cancer genetics research. Cancer Epidemiology, Biomarkers & Prevention, 15(7), 1355–1359.
Green, D., Cushman, M., Dermond, N., Johnson, E. A., Castro, C., Arnett, et al. (2006). Obtaining informed consent for genetic studies: the multiethnic study of atherosclerosis. American Journal of Epidemiology, 164(9), 845–851. doi:10.1093/aje/kwj286
Jacobi, C. E., de Bock, G. H., Siegerink, B., & van Asperren, C. J. (2009). Differences and similarities in breast cancer risk assessment models in clinical practice: which model to choose? Breast Cancer Research and Treatment, 115(2), 381–390.
Katapodi, M., Lee, K., Facione, N., & Dodd, M. (2004). Predictors of breast cancer risk and the relation between perceived risk and breast cancer screening: a meta-analysis. Preventative Medicine, 38(4), 388–402. doi:10.1016/j.ypmed.2003.11.012.
Kostev, K., Waehlert, L., Jockwig, A., Jockwig, B., & Hadji, P. (2014). Physicians’ influence on breast cancer patient compliance. GMS German Medical Science, 12, Doc03. doi:10.3205/000188.
Lerman, C., Lustbader, E., Rimer, B., Daly, M., Miller, S., Sands, C., et al. (1995). Effects of individualized breast cancer risk counseling: a randomized trial. Journal of the National Cancer Institute, 87(4), 286–292. doi:10.1093/jnci/87.4.286.
McCaul, K. D., Branstetter, A. D., Schtoeder, D. M., & Glasgow, R. E. (1996). What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychology, 15(6), 423–429. doi:10.1037/0278-6133.15.6.423.
McQuillan, G. M., Porter, K. S., Agelli, M., & Kington, R. (2003). Consent for genetic research in a general population: the NHANES experience. Genetics in Medicine, 5(1), 35–42.
Mellon, S., Gold, R., Janisse, J., Cichon, M., Tainsky, M. A., Simon, M. S., et al. (2008). Risk perception and cancer worries in families at increased risk of familial breast/ovarian cancer. Psycho-Oncology, 17(8), 756–766. doi:10.1002/pon.1370.
Mezuk, B., Eaton, W. W., & Zandi, P. (2008). Participant characteristics that influence consent for genetic research in a population-based survey: the Baltimore epidemiologic catchment area follow-up. Community Genetics, 11(3), 171–178. doi:10.1159/000113880.
Moorman, P. G., Skinner, C. S., Evans, J. P., Newman, B., Sorenson, J. R., Calingaert, B., et al. (2004). Racial differences in enrollment in a cancer genetics registry. Cancer Epidemiology, Biomarkers & Prevention, 13(8), 1349–1353.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2014a). Breast Cancer Risk Reduction. (Version 1.2014). Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed 23 Mar 2016.
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2014b). Genetic/Familial High-Risk Assessment: Breast and Ovarian. (Version 1.2014). Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed 23 Mar 2016.
Royak-Schaler, R., Klabunde, C. N., Greene, W. F., Lannin, D. R., DeVellis, B., Wilson, K. R., et al. (2002). Communicating breast cancer risk: patient perceptions of provider discussions. Medscape Women’s Health, 7(2), 2.
Saslow, D., Boetes, C., Burke, W., Harms, S., Leach, M. O., Lehman, C. D., et al. (2007). American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: a Cancer Journal for Clinicians, 57(2), 75–89.
Scherer, L. D., Ubel, P. A., McClure, J., Green, S. M., Alford, S. H., Holtzman, L., et al. (2013). Belief in numbers: when and why women disbelieve tailored breast cancer risk statistics. Patient Education and Counseling, 92(2), 253–259. doi:10.1016/j.pec.2013.03.016.
Shih, T. H., & Fan, X. (2009). Comparing response rates in e-mail and paper surveys: a meta-analysis. Educational Research Review, 4(1), 26–40.
Siegel, R., Ma, J., Zou, Z., & Jemal, A. (2014). Cancer statistics, 2014. CA: a Cancer Journal for Clinicians, 64, 9–29. doi:10.3322/caac.21208.
Tchou, J., & Morrow, M. (2003). Available models for breast cancer risk assessment: how accurate are they? Journal of the American College of Surgeons, 197(6), 1029–1035.
Acknowledgements
The authors would like to thank Carolyn Taylor and Michelle Hall for their editing contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nichole A. Morman, Lindsey Byrne, Christy Collins, Kelly Reynolds, and Jeffrey G. Bell declare they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all women for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Rights and permissions
About this article
Cite this article
Morman, N.A., Byrne, L., Collins, C. et al. Breast Cancer Risk Assessment at the Time of Screening Mammography: Perceptions and Clinical Management Outcomes for Women at High Risk. J Genet Counsel 26, 776–784 (2017). https://doi.org/10.1007/s10897-016-0050-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-016-0050-y