Abstract
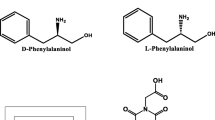
Chirality plays a pivotal role in drugs, agrochemicals and food additives et al. The enantiomers of a chiral molecule often show huge difference in bioactivity, metabolism, and toxicity et al. thereby, the recognition of chiral molecules shows an increasingly important priority. In this paper, a novel method for chiral fluorescence recognition based on anthracene fluorescent dyes (AD) ⊂ water-soluble pillar[5] arene containing phosphonic acid group (PWP[5]) is developed. The AD as guest molecule can complex with PWP [5] to form 1:1 AD ⊂ PWP[5] assembly, and this assembly can be further used as a fluorescent probe to identify D/L-phenylalanine and D/L-phenylalaninol by fluorescent titration. The fluorescence intensity of the assembly was significantly reduced for D-phenylalanine and D-phenylalaninol, while L-phenylalanine or L-phenylalaninol was added to AD ⊂ PWP[5] assembly, the fluorescence intensity of the assembly almost unchanged. Hence, the chiral recognition based on assembly between the achiral fused ring fluorescent dye and achiral PWP[5] was developed.














Similar content being viewed by others
Data Availability Statement
Data sharing does not apply to this article Because no database was generated or analyzed during the current research period.
Code Availability
Not applicable.
References
Rei UT et al (2003) Evolution: single-gene speciation by left–right reversal. Nature 425:679
Ma W, Xu L, André et al (2017) Chiral inorganic nanostructures. Chem Rev 117:8041–8093
Randová A, Bartovská L, Hovorka, et al (2015) Sorption of enantiomers and alcohols into nafion and the role of air humidity in the experimental data evaluation. Sep Purif Technol 144:232–239
Kowalski TW, Fraga LR, Tovo-Rodrigues L et al (2017) Angiogenesis-related genes and thalidomide teratogenesis in humans: an approach on genetic variation and review of past in vitro studies. Reprod Toxicol 70:133–140
Miller L, Lei Y (2020) Chiral separation of underivatized amino acids in supercritical fluid chromatography with chiral crown ether derived column. Chirality 32:981–989
Iwanaga T, Nakamoto R, Yasutake M et al (2006) Cyclophanes within cyclophanes: the synthesis of a pyromellitic diimide-based macrocycle as a structural unit in a molecular tube and its inclusion phenomena. Angew Chem Int Ed 118:3725–3729
Flores-Sánchez R, Gámez F, Lopes-Costa T et al (2020) A calixarene promotes disaggregation and sensing performance of carboxyphenyl porphyrin films. ACS Omega 5:6299–6308
Liu YH, Zhang YM, Yu HJ et al (2020) Cucurbituril-based biomacromolecular assemblies. Angew Chem Int Ed 60:3870–3880
Ogoshi T, Kanai S, Fujinami S et al (2008) Para-bridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host-guest property. J Am Chem Soc 130:5022–5023
Ilisz I, Berkecz R, Péter A (2008) Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. J Pharm Biomed Anal 47:1–15
Mala Z, Gebauer Petr, Bocek et al (2017) Recent progress of sample stacking in capillary electrophoresis (2014–2016). Electrophoresis: The Official J Int Electr Soc 38:20–32
Valdez CA, Leif RN, Hok S et al (2017) Analysis of chemical warfare agents by gas chromatography-mass spectrometry: methods for their direct detection and derivatization approaches for the analysis of their degradation products. Rev Anal Chem 37:1–22
Tang Q, Zhao L, Xie J et al (2019) Deviations from beer’s law in electronic absorption and circular dichroism: detection for enantiomeric excess analysis. Chirality 31:492–501
Pan Q, He Z, Xia D et al (2018) Fluorescent determination of succinylcholine chloride by naphthalimide / stilbazolium dye CP5A. J Fluoresc 28:581–587
Chen X, Hu N, Wei H et al (2020) Chiral fluorescent recognition by naphthalimide dyes ⊂ Cucurbit[7] uril Assembly. J Fluoresc 30:679–685
Hu XY, Liu X et al (2016) Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem Mater 28:3778–3788
Acknowledgements
The authors gratefully appreciated the support from Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21-1079). The authors also thanks to the support of the equipment provided by the college of chemistry and molecular engineering, Nanjing Tech University.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21406108). The research was conducted under financial support from National/Jiangsu College Students Innovation and Entrepreneurship Training Program, which was gratefully appreciated. The financial support from Nanjing Tech University was also gratefully appreciated.
Author information
Authors and Affiliations
Contributions
YC, KL, YL, and JC completed all experimental work. YC analyzed the results and wrote papers, HW proposed concepts, designed assemble model ideas, and provided supervision and completed review.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
There is no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Lai, K., Cai, J. et al. Chiral Fluorescence Recognition by Anthracene Fluorescent Dyes ⊂ Water-Soluble Pillar[5] arene containing Phosphonic Acid Group (PWP[5]). J Fluoresc 32, 983–992 (2022). https://doi.org/10.1007/s10895-022-02908-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02908-3