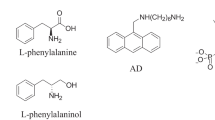
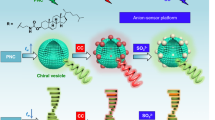
Abstract
Chirality plays a very important role in medicine, biochemistry and other fields. Because the enantiomers of chiral drugs often show different pharmacology activity, metabolism, and toxicity, therefore, the recognition of chiral molecules is very important, and has become a hot spot and frontier of modern chemical research. In this paper, a new method for recognizing chiral molecular based on naphthalimide dye(NA)⊂cucurbit[5]uril(CB[7]) assembly is developed. NA as guest can be combined with the host CB[7] to form a 1:1 NA⊂CB[7] assembly. Furthermore, this assembly was used as a fluorescent probe to recognize D/L-phenylalanine and D/L-phenylalaninol by fluorescence titration. When D-phenylalanine or D-phenylalaninol was added to NA⊂CB[7] assembly, the fluorescent intensity of assembly was partially quenched, but when L-phenylalanine or L-phenylalaninol was added to NA⊂CB[7], the fluorescence intensity of the assembly almost unchanged. Herein, chiral recognition platform based on achiral NA⊂achiral CB[7] was constructed.










Similar content being viewed by others
References
Miner-Williams W, Moughan PJ, Fuller MF (2009) Endogenous components of digesta protein from the terminal ileum of pigs fed a casein-based diet[J]. J Agric Food Chem 57(5):2072–2078
Zhang XX, Bradshaw JS, Izatt RM (1997) Enantiomeric recognition of amine compounds by chiral macrocyclic receptors[J]. Chem Rev 97(8):3313–3362
Korbel GA, Gojko Lalic A, Shair MD (2001) Reaction microarrays: a method for rapidly determining the enantiomeric excess of thousands of samples[J]. J Am Chem Soc 123(2):361–362
Eelkema R, Delden RAV, Feringa BL (2004) Direct visual detection of the stereosel ectivity of a catalytic reaction[J]. Angew Chem Int Ed 43(38):50 13-5016
Ogoshi T, Kanai S, Fujinami S, Yamagishi TA, Nakamoto Y (2008) para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. Am Chem Soc 130:5022–5023
Pedersen CJ (2010) The discovery of crown ethers (noble lecture). Angew Chem Int Ed 27:1021–1027
Iwanaga T, Nakamoto R, Yasutake M et al (2006) Cyclophanes within Cyclop hanes: The synthesis of a pyromellitic diimide-based macrocycle as a structural unit in a molecular tube and its inclusion phenomena[J]. Angew Chem Int Ed 45(22):3643–3647
Böhmer V (2010) Calixarenes, Macrocycles with (Almost) Unlimited Possibilities[J]. Angew Chem Int Ed 34(7):713–745
Mandadapu V, Day AI (2014) Ghanem A. Cucurbituril: chiral ap-plications. Chirality 26:712–723
Freeman WA, Mock WL, Shih NY (1981) Cucurbituril. J Am Chem Soc 103:7367–7368
Ilisz I, Berkecz R, Péter A (2008) Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: A review[J]. J Pharm Biomed Anal 47(1):1–15
Desiderio C, Fanali S (1998) Chiral analysis by capillary electrophoresis using antibiotics as chiral selector[J]. J Chromatogr A 818(2):281–282
Schurig V (2002) Practice and theory of enantioselective complexation gas chromatography[J]. J Chromatogr A 965(1–2):315–356
Tang Q et al (2019) Deviations from Beer’s law in electronic absorption and circular dichroism: Detection for enantiomeric excess analysis. Chirality 31(7):492–501
Li J, Gu X, Yuan X et al (2016) Cis/trans fluorescent recognition by naphthalimide dyes⊂CB [7] assembly[J]. J Fluoresc 26(4):1219–1224
Jiao DZ, Scherman OA (2012) Isolation of cucurbit[n]uril homo-logues with imidazolium salts in a recyclable manner. Green Chem 14(9):2445–2449
Pan Q, He Z, Xia D et al (2018) Fluorescent determination of succinylcholine chloride by naphthalimide/ stilbazolium dye ⊂ CP5A[J]. J Fluoresc 28(2):581–587
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21406108).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Hu, N., Wei, H. et al. Chiral Fluorescent Recognition by Naphthalimide. J Fluoresc 30, 679–685 (2020). https://doi.org/10.1007/s10895-020-02539-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02539-6