Abstract
The difference between venous and arterial carbon dioxide pressure (pCO2 gap), has been used as a diagnostic and prognostic tool. We aimed to assess whether perioperative pCO2 gaps can predict postoperative complications. This was a secondary analysis of a multicenter RCT comparing goal-directed therapy (GDT) to standard care in which 464 patients undergoing high-risk elective abdominal surgery were included. Arterial and central venous blood samples were simultaneously obtained at four time points: after induction, at the end of surgery, at PACU/ICU admission, and PACU/ICU discharge. Complications within the first 30 days after surgery were recorded. Similar pCO2 gaps were found in patients with and without complications, except for the pCO2 gap at the end of surgery, which was higher in patients with complications (6.0 mmHg [5.0–8.0] vs. 6.0 mmHg [4.1–7.5], p = 0.005). The area under receiver operating characteristics curves for predicting complications from pCO2 gaps at all time points were between 0.5 and 0.6. A weak correlation between ScvO2 and pCO2 gaps was found for all timepoints (ρ was between − 0.40 and − 0.29 for all timepoints, p < 0.001). The pCO2 gap did not differ between GDT and standard care at any of the selected time points. In our study, pCO2 gap was a poor predictor of major postoperative complications at all selected time points. Our research does not support the use of pCO2 gap as a prognostic tool after high-risk abdominal surgery. pCO2 gaps were comparable between GDT and standard care. Clinical trial registration Netherlands Trial Registry NTR3380.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pCO2 gap is the difference between venous and arterial carbon dioxide pressure and might be used for diagnostic, prognostic, or therapeutic purposes. First, an association between pCO2 gap and postoperative complications was found in patients undergoing major abdominal surgery; pCO2 gap was found to be higher in the proportion of patients suffering from postoperative complications [1]. In patients with septic shock, a persistently high pCO2 gap was associated with worse outcomes [2] and was found to be a modest predictor of mortality [3]. Second, pCO2 gaps reflect the adequacy of cardiac output and tissue perfusion [4]. An inverse relationship exists between cardiac output and pCO2 gap, and an increase in pCO2 gap to more than 6 mmHg is considered abnormal. Additionally, pCO2 gaps have been shown to reliably estimate cardiac index in a perioperative setting [5]. Third, pCO2 gaps may be used as a target in a goal-directed therapy (GDT) protocol. GDT uses preset hemodynamic targets to guide hemodynamic interventions, such as vasopressors, inotropes, and fluids [6]. GDT can focus on any given hemodynamic variable; however, it is recommended to use variables representing blood flow [6]. It has been shown that perioperative GDT reduces morbidity and mortality [7, 8].
The majority of studies investigating pCO2 gap have been conducted in critically-ill patients and the evidence for the use of pCO2 gaps in the perioperative setting is limited [9]. The primary aim of this study was to assess the association and predictive value of the perioperative pCO2 gap with a composite outcome of major postoperative complications using patient data from a randomized controlled trial comparing GDT versus standard care in patients undergoing elective high-risk abdominal surgery [10]. The secondary aim of this study was to assess the correlation between ScvO2 and pCO2 gap. Central venous oxygen saturation (ScvO2) is a representation of oxygen delivery and demand and can be used to assess global tissue oxygenation [11], similar to pCO2 gap, which reflects adequacy of cardiac output and tissue perfusion. Yet, the correlation between the two has not been assessed in patients undergoing elective high-risk abdominal surgery. Finally, pCO2 gaps were compared between standard care and GDT as a measure of adequacy of cardiac output, since actual cardiac output measurements were not available in this study.
2 Materials & methods
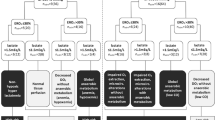
This study concerned a secondary analysis of a multicenter randomized controlled trial comparing the incidence of major complications in the first 30 days between a GDT and standard care in patients undergoing elective, high-risk abdominal surgery [10]. The study was approved by all necessary ethical review boards and written informed consent was obtained before any study procedures were conducted. This manuscript adheres to the CONSORT reporting guidelines [12]. Patients aged 18 years or older were included when they were undergoing elective, high-risk abdominal surgery. Exclusion criteria were emergency surgery, aortic valve insufficiency grade > 1, cardiac arrhythmias, contraindications to the passive leg raising test, and indication for invasive cardiac output monitoring during surgery. A CONSORT flow diagram can be found in Fig. 1.
2.1 Study procedures
The detailed study protocol, randomization sequence, and primary study outcomes have been previously published [10, 13]. In all patients, an arterial and central venous catheter was inserted. The GDT group was additionally monitored using arterial waveform analysis (FloTrac - Vigileo, Edwards Lifesciences, Irvine, CA, USA) and treated according to a cardiac index (CI) targeted GDT algorithm [10]. The GDT algorithm was initiated after anesthesia induction and continued for a maximum of 24 h or until PACU/ICU discharge, whichever occurred first. Stopping criteria were arrhythmia, (suspected) myocardial ischemia, and cardiac decompensation.
Arterial and central venous blood samples were simultaneously obtained at four time points: after induction, at the end of surgery, at PACU/ICU admission, and at PACU/ICU discharge. All blood samples were directly analyzed per local practice.
2.2 Outcomes
The primary outcome was to assess the association between pCO2 gap and a composite outcome of major complications. The composite outcome of major complications, as considered by the Accordion Severity Grading system [14], consisted of the following: death, cardiac arrest, acute myocardial infarction, acute pulmonary edema, cerebrovascular accident, prolonged mechanical ventilation, pulmonary embolism, pneumonia, respiratory insufficiency, acute kidney injury, anastomotic leakage, other gastro-intestinal complications, wound infection and severe sepsis [10]. Therefore, all subjects from the original study who did not have at least one pCO2 gap value were excluded from this analysis, resulting in 464 remaining subjects of the original 482 subjects. pCO2 gaps larger than + 30.0 mmHg or smaller than − 7.5 mmHg were considered artifacts and were removed [15].
Additionally, arterial and central venous oxygen saturation, lactate, and pH were compared between both groups. Arterial oxygen saturation measurements, which were lower than their venous counterpart, were considered artifacts, and both the venous and arterial measurements were removed (n = 2). An additional 12 venous oxygen saturation values were removed as they were in the range of 5 to 7% and were considered artifacts. For lactate, three erroneous entries were removed, one was a negative value and the other two were below the detectable limit. Subsequently, the correlation between pCO2 gaps and central venous oxygen saturation was assessed.
Last, the difference in perioperative pCO2 gaps between GDT and standard care were assessed as a measure of the adequacy of cardiac output.
2.3 Statistical analysis
Continuous variables were presented as mean and standard deviation (SD) or median and interquartile ranges (IQR) when indicated. Normality was visually assessed using Q-Q plots. Categorical data were presented as numbers and percentages. Continuous data were compared using the Mann-Whitney-U test since none of the continuous data met the parametric assumption. Categorical data were compared using Chi-square or Fisher’s exact test when indicated. A Spearman correlation was used to assess the correlation between pCO2 gap and ScvO2. A logistic regression analysis was performed to assess the association between pCO2 gap and major complications. Subsequently, Receiver Operating Characteristics (ROC) curves were plotted and the areas under the ROC (AUROC) curve were assessed. Missing data were coded as missing, and no imputation was used. A p-value of 0.05 was considered statistically significant. All analyses were performed using RStudio (version 1.4.1106, RStudio, Vienna, Austria).
3 Results
3.1 Patient characteristics
A total of 464 patients were included in this secondary analysis (Fig. 1). Eighteen patients were excluded from the original analysis since pCO2 gaps for all four time points were missing. The age in the GDT group was 65 [59–73] years versus 67 [61–75] years in the control group. Other patient characteristics, e.g., gender, body mass index, American Society of Anesthesiology physical status, comorbidities, and type of surgery, can be found in Table 1.
3.2 Association between pCO2 gaps and major complications
Two hundred patients (43%) suffered from major complications. pCO2 gaps after induction of anesthesia, PACU/ICU admission, and PACU/ICU discharge were similar for patients with and without postoperative complications (Table 2; Fig. 2). At the end of surgery, the patients with major complications had a higher pCO2 gap than those without major complications (6.0 mmHg [5.0–8.0] vs. 6.0 mmHg [4.1–7.5], p = 0.005).
pCO2 gaps at different timepoints: A After induction, B At the end of surgery, C At PACU/ICU admission, D At PACU/ICU discharge. The boxes represent the 25th through 75th percentile including the median. The whiskers represent the highest or lowest values to a maximum of 1.5 times the IQR, otherwise the data point is considered an outlier (black dots)
Logistic regression showed an association between pCO2 gap at the end of surgery and major complications (χ2(1) = 5.77, p = 0.016 (Table 3)) with an odds ratio of 1.08 (95%CI 1.01–1.16). The AUROCs for predicting postoperative complications were 0.508 (95%CI 0.454–0.563) for pCO2 gap after induction, 0.578 (95%CI 0.524–0.633) at the end of surgery, 0.524 (95%CI 0.468–0.580) at PACU/ICU admission, and 0.499 (95%CI 0.438–0.560) at PACU/ICU discharge. Since all AUROCs were between 0.5 and 0.6, cut-off values were not further explored.
3.3 Correlation central venous oxygen saturation and pCO2 gaps
A weak correlation was found between ScvO2 and pCO2 gaps at all four time points (after induction ρ = -0.39 (p < 0.001), at the end of surgery ρ = −0.29 (p < 0.001), at PACU/ICU admission ρ= −0.39 (p < 0.001), and at PACU/ICU discharge ρ= −0.40 (p < 0.001), Fig. 3).
Correlation between pCO2 gap and ScvO2. pCO2 gap = difference between venous and arterial carbon dioxide pressure. ScvO2 = central venous oxygen saturation A Correlation between pCO2 gap and ScvO2 obtained after induction of anesthesia. B Correlation between pCO2 gap and ScvO2 obtained at the end of surgery. C Correlation between pCO2 gap and ScvO2 obtained at PACU/ICU admission. D Correlation between pCO2 gap and ScvO2 obtained at PACU/ICU discharge
3.4 Blood gas analyses GDT vs. control
Arterial pCO2, venous pCO2, and pCO2 gaps at all time points did not significantly differ between GDT and standard care (Table 4). Central venous oxygen saturation after surgical closure was significantly higher in the GDT group compared to the control group (83% [79–87%] vs. 82% [77–86%], p = 0.032, Table 4). Lactate was higher in the GDT group compared to the control group after surgical closure (2.1 mmol L−1 [1.3–3.0 mmol L−1] vs. 1.8 mmol L−1 [1.3–2.6 mmol L−1], p = 0.046, Table 4). The pH was similar between the two groups at any of the time points (Table 4).
4 Discussion
This study is the largest multicenter trial to date concerning the prognostic ability of pCO2 gaps in a non-cardiac surgical population. We found that patients who suffered from major postoperative complications had a statistically significantly higher pCO2 gap at the end of surgery than patients without major complications. However, this difference cannot be considered clinically significant. Furthermore, pCO2 gap was a poor predictor of major postoperative complications at any of the selected time points. The GDT group did however, have higher ScvO2 and higher lactate at the end of surgery than the control group. Perioperative pCO2 gaps were similar for the GDT and control group in patients undergoing high-risk abdominal surgery.
The patients who suffered from major postoperative complications had a higher pCO2 gap at the end of surgery (6.0 mmHg [5.0–8.0]) compared to patients without major complications (6.0 mmHg [4.1–7.5], p = 0.005). Although the small difference in pCO2 gap between both groups was statistically significant, it was not considered clinically relevant and may also be explained by multiple testing. A difference in pCO2 gap observations was reported in several studies for patients with and without complications after elective surgery. In the first study of 70 patients undergoing major abdominal surgery, the mean pCO2 gap was found to be higher in patients suffering from complications (n = 24, 34%) compared to those patients without complications (7.8 ± 2 mmHg vs. 5.6 ± 2 mmHg, p < 10−6) [1]. pCO2 gap values were determined at baseline and then hourly until discharge from the PACU. In a second study performed in 115 high-risk surgical patients, the mean pCO2 gap was also higher in patients who developed postoperative complications (n = 78, 68%) compared to patients without complications (8.7 ± 2.8 mmHg vs. 5.1 ± 2.6 mmHg, p < 0.001) [16]. The studied population underwent elective major abdominal and vascular surgery and was admitted to the ICU, pCO2 gap values were obtained at baseline and then hourly until discharge from the PACU. A third study included 90 patients undergoing major abdominal surgery with scheduled admission to the ICU. The median intraoperative pCO2 gap was higher in patients with complications (n = 39, 43%) compared to patients without complications (6.5 mmHg [5.5–7.3] vs. 5.0 mmHg [3.9–5.8], p < 0.001) [17]. The median postoperative pCO2 gap was higher in patients with complications compared to patients without complications (6.8 mmHg [5.7–8.7] vs. 6.0 mmHg [4.7–7.1], p = 0.03). pCO2 gap values were obtained every two hours from baseline to the end of surgery, at ICU admission, and 12 and 24 h after ICU admission.
For comparison purposes, we pooled pCO2 gaps at all time points showing similar median pCO2 gaps for patients with complications (n = 139, 46%) compared to patients without complications (6.0 mmHg [5.3-7.0] vs. 5.8 mmHg [4.5-7.0], p = 0.078). pCO2 gap of patients with complications in our population was low compared to the three previously mentioned studies. Our population consisted of a broader mix of surgical procedures and were not necessarily postoperatively admitted to the ICU. It may be that a relatively healthier population has undergone a relatively less invasive surgery which may partly explain the lack of difference in pCO2 gap in our population.
pCO2 gap was a poor predictor of major postoperative complications at any of the given time points in our population. Better discrimination was found in the previously mentioned studies as a result of a larger difference in pCO2 gap between the groups with and without complications [1, 16, 17].
Pitfalls exist in the interpretation of pCO2 gaps. A few mechanisms that affect pCO2 gap are the Haldane effect and hyperventilation [18]. Thus, it was suggested that only variations of > 2 mmHg should be considered real variations [19]. pCO2 gap obtained at the end of surgery in patients with and without complications did not exceed this threshold.
The GDT group had a significantly higher ScvO2 at the end of surgery, which could be related to the interventions as indicated by the treatment algorithm. In addition, higher lactate was found in this group at the end of surgery. This could be caused by the administration of higher volumes of packed red blood cells (not reported here) [10]. With a longer storage time of packed red blood cells lactate concentrations increase [20, 21], although we did not collect this data. For ScvO2 as well as lactate, the statistical difference cannot be considered a clinically relevant difference.
We did not find a difference in perioperative pCO2 gaps between the GDT and control group in patients undergoing high-risk abdominal surgery. Since cardiac output measurements were absent in the control group of the study, we felt the need to compare adequacy of cardiac output between GDT and standard care by comparing perioperative pCO2 gaps as a measure of cardiac output. We therefore hypothesize that both GDT and standard clinical practice led to an adequate cardiac output in our studied population. This may partly help explain why we did not find a difference in major and minor complications and hospital and PACU/ICU length of stay in the original study [10].
Our study has several limitations. First, the control group did not receive additional hemodynamic monitoring and therefore cardiac output was not available. This forced us to use pCO2 gap solely as a surrogate for cardiac output without being able to assess the relationship between pCO2 gap and cardiac output for this group. Second, it is recommended to use mixed venous pCO2 to calculate pCO2 gap instead of central venous pCO2 [4, 22]. However, since mixed and central venous pCO2 have a good agreement, central venous pCO2 can be used for this purpose as long as it is not used interchangeably during treatment [22]. Third, we did not obtain data on variables that could have affected pCO2 gaps, e.g., hemodilution and body temperature. Fourth, more frequent measurements would have minimized the effect of outliers.
So far, the evidence is inconsistent with the prognostic value of pCO2 gaps in a surgical population. Recently, the first trial incorporating pCO2 gap in a GDT treatment algorithm has been published [23]. One hundred ASA I/II patients were included and allocated to GDT with ScvO2 as a primary target and pCO2 gap as a secondary target or to a control group with only arterial blood gas analysis available. No difference was found between the groups in postoperative organ dysfunction (defined by SOFA scores), although the GDT group had a lower length of ICU stay (1.52 ± 0.82 vs. 2.18 ± 1.08 days). Future studies should focus on clarifying the prognostic abilities of pCO2 gap by increasing measurement frequencies, including high-risk patients, and recording factors that influence pCO2 gaps.
5 Conclusion
In conclusion, an association was found between pCO2 gap at the end of surgery and major postoperative complications, but pCO2 gap was a poor predictor of major postoperative complications at all given time points in our population. Therefore, the use of pCO2 gap as a prognostic tool in patients undergoing high-risk abdominal surgery is limited. Moreover, we did not find a difference in of pCO2 gap between a GDT and a control group of patients undergoing high risk abdominal surgery, indicating that both patient groups were sufficiently hemodynamically optimized.
Data availability
Dataset are not publicly available.
References
Futier E, Robin E, Jabaudon M, Guerin R, Petit A, Bazin JE, et al. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk Surgery. Crit Care. 2010;14:R193. https://doi.org/10.1186/cc9310.
Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, Tafur JD, Gutiérrez A, García AF, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17:R294. https://doi.org/10.1186/cc13160.
Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101:509–15. https://doi.org/10.1378/chest.101.2.509.
Ltaief Z, Schneider AG, Liaudet L. Pathophysiology and clinical implications of the veno-arterial PCO2 gap. Crit Care. 2021;25:318. https://doi.org/10.1186/s13054-021-03671-w.
Tsaousi GG, Karakoulas KA, Amaniti, Ekaterini N, Soultati ID, Zouka, Maria D, Vasilakos DG. Correlation of central venous–arterial and mixed venous–arterial carbon dioxide tension gradient with cardiac output during neurosurgical procedures in the sitting position. Eur J Anaesthesiol. 2010;27:882–9. https://doi.org/10.1097/EJA.0b013e32833d126f.
Kaufmann T, Saugel B, Scheeren TWL. Perioperative goal-directed therapy–what is the evidence? Best Pract Res Clin Anaesthesiol. 2019;33:179–87. https://doi.org/10.1016/j.bpa.2019.05.005.
Chong MA, Wang Y, Berbenetz NM, McConachie I, et al. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes? Eur J Anaesthesiol. 2018;35:469–83. https://doi.org/10.1097/EJA.0000000000000778.
Teboul J, Mercat A, Lenique F, Berton C, Richard C. Value of the venousarterial PCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med. 1998;26:1007–10. https://doi.org/10.1097/00003246-199806000-00017.
Huette P, Ellouze O, Abou-Arab O, Guinot PG. Venous-to-arterial pCO2 difference in high-risk surgical patients. J Thorac Dis. 2019;11:1551–7. https://doi.org/10.21037/jtd.2019.01.109.
de Waal EEC, Frank M, Scheeren TWL, Kaufmann T, de Korte DJD, Cox B, et al. Perioperative goal-directed therapy in high-risk abdominal Surgery. A multicenter randomized controlled superiority trial. J Clin Anesth. 2021;75:110506. https://doi.org/10.1016/j.jclinane.2021.110506.
Van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M. Clinical review: use of venous oxygen saturations as a goal—a yet unfinished puzzle. Crit Care. 2011;15:232. https://doi.org/10.1186/cc10351.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
Montenij L, De Waal E, Frank M, et al. Influence of early goal-directed therapy using arterial waveform analysis on major Complications after high-risk abdominal Surgery: study protocol for a multicenter randomized controlled superiority trial. Trials. 2014;15:360. https://doi.org/10.1186/1745-6215-15-360.
Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of Surgical Complications. Ann Surg. 2009;250:177–86. https://doi.org/10.1097/SLA.0b013e3181afde41.
Shastri L, Boulain T, Rees SE, Thomsen LP. Comparison of two methods for converting central venous values of acid-base status to arterial values in critically ill patients. Comput Methods Programs Biomed. 2021;203:106022. https://doi.org/10.1016/j.cmpb.2021.106022.
Robin E, Futier E, Pires O, Fleyfel M, Tavernier B, Lebuffe G, et al. Central venous-to-arterial carbon dioxide difference as a prognostic tool in high-risk surgical patients. Crit Care. 2015;19:227. https://doi.org/10.1186/s13054-015-0917-6.
Guilherme E, Delignette M-C, Pambet H, Lebreton T, Bonnet A, Pradat P, et al. PCO2 gap, its ratio to arteriovenous oxygen content, ScvO2 and lactate in high-risk abdominal surgery patients: an observational study. Anaesth Crit Care Pain Med. 2022;41:101033. https://doi.org/10.1016/j.accpm.2022.101033.
Saludes P, Proença L, Gruartmoner G, Enseñat L, Pérez-Madrigal A, Espinal C, et al. Central venous-to-arterial carbon dioxide difference and the effect of venous hyperoxia: a limiting factor, or an additional marker of severity in shock? J Clin Monit Comput. 2017;31:1203–11. https://doi.org/10.1007/s10877-016-9954-1.
Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D. Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med. 2016;5:47. https://doi.org/10.5492/wjccm.v5.i1.47.
Ratcliffe J, Elliott M, Wyse R, Hunter S, Alberti K. The metabolic load of stored blood. Implications for major transfusions in infants. Arch Dis Child. 1986;61:1208–14. https://doi.org/10.1136/adc.62.11.1199.
Schroeder TH, Hansen M. Effects of fresh versus old stored blood in the priming solution on whole blood lactate levels during paediatric cardiac Surgery. Perfusion. 2005;20:17–9. https://doi.org/10.1191/0267659105pf784oa.
Van Beest PA, Lont MC, Holman ND, Loef B, Kuiper MA, Boerma EC. Central venous-arterial pCO2 difference as a tool in resuscitation of septic patients. Intensive Care Med. 2013;39:1034–9. https://doi.org/10.1007/s00134-013-2888-x.
Kumar LHN, Tripathy S, Kumar Das P. Central venous-to-arterial CO2 difference–assisted goal-directed hemodynamic management during major surgery—a randomized controlled trial. Anesth Analg. 2022;134:1010–20. https://doi.org/10.1213/ane.0000000000005833.
Acknowledgements
We thank Gerda Kuipers, Rob Spanjersberg, Ada van Kampen and Gea Mulder, as members of the trial agencies of the respective hospitals for valuable help to recruit patients and collect patient data.
Funding
This work was supported by an unrestricted grant of the Janivo Stichting, Zeist, Netherlands.
Author information
Authors and Affiliations
Contributions
EECW, LMM, TWLS and WB contributed to the study conception and design. Material preparation, data collection and analysis were performed by INK, TK and TWLS. The first draft of the manuscript was written by INK and TK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Wolfgang Buhre has received honoraria for lectures and was a consultant for both Pulsion Medical Systems/Maquet and Edwards Lifesciences. Thomas Scheeren received research grants and honoraria from Masimo Inc. (Irvine, CA, USA) for consulting and lecturing (all payments made to institution) and Edwards Lifesciences (Irvine, CA, USA), is currently employed by Edwards Lifesciences. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This trial was approved by the Medical Research Ethics Committee of the University Medical Centre Utrecht, The Netherlands (10–173/O) and registered in the Dutch Trial Register (registration number NTR3380) on April 3rd 2012. Local approval was obtained in each participating center. This trial was approved by the Medical Research Ethics Committee of the University Medical Centre Utrecht, The Netherlands (10–173/O) and registered in the Dutch Trial Register (registration number NTR3380) on April 3rd 2012. Local approval was obtained in each participating center.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Keijzer, I.N., Kaufmann, T., de Waal, E.E. et al. Can perioperative pCO2 gaps predict complications in patients undergoing major elective abdominal surgery randomized to goal-directed therapy or standard care? A secondary analysis. J Clin Monit Comput 38, 469–477 (2024). https://doi.org/10.1007/s10877-023-01117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-01117-y