Abstract
Fluid responsiveness prediction is difficult during cardiac surgery. The micro-fluid challenge (micro-FC; rapid central infusion of 50 ml) and the extrasystolic method utilising post-extrasystolic preload increases may predict fluid responsiveness. Two study windows during coronary artery bypass graft surgery were defined, 1: After anaesthesia induction until surgical incision, 2: Left internal mammarian artery surgical preparation period. Each window consisted of 10–15 min observation for extrasystoles before a micro-FC was performed, after which a traditional fluid challenge (FC) was performed (5 ml/kg). Extrasystolic and micro-FC induced changes in hemodynamic variables were derived as predictors of fluid responsiveness defined as stroke volume increasing > 10% following FC. 61 patients were studied. Post-ectopic changes in pulse pressure (PP) predicted fluid responsiveness with receiver operating characteristic area (AUC) of 0.69 [CI 0.40;0.97] in the first study window and 0.64 [0.44;0.86] in the second window. Other post-ectopic predictors such as pre-ejection period (PEP) and systolic blood pressure (SBP) had similar or lower AUCs. Heart rate was 52.9 (SD ±8.4) min− 1 and 53.6 (± 8.8) min− 1 in the two study windows. Micro-FC induced changes in PEP had AUC of 0.74 [0.57;0.90] in the first window and 0.60 [0.40;0.76] in the second window. Correcting micro-FC induced changes in PEP for the micro-FC induced changes in heart rate had AUCs of 0.84 [0.70;0.97] in the first window and 0.63 [0.47;0.79] in the second window. The investigated methods revealed insufficient validity during cardiac surgery. RR interval corrected changes during a micro-FC should be investigated further. Trial registration Clinicaltrials.gov: NCT03002129.
Similar content being viewed by others
1 Introduction
Fluid responsiveness prediction during general anaesthesia has historically moved from using static preload variables, such as central venous pressure (CVP), to preferably using so-called dynamic variables. The latter utilise the fact that preload varies during positive pressure ventilation and, as a consequence, that the “patient’s heart is surfing” the curvilinear Frank-Starling curve [1]. The magnitude of corresponding heart work variations (reflected in e.g. pulse pressure variation, PPV [2]) discloses the Frank-Starling curve slope and in diverse patient groups, dynamic variables have repeatedly demonstrated their ability to predict fluid responsiveness in this specific controlled-ventilated population [3, 4].
Unfortunately, dynamic variables have several physiological limitations precluding its use in all patients [5]. The most often encountered problem is that patients are not ventilated with tidal volumes large enough to evoke the heart–lung interactions needed for the dynamic variables to be reliable [6, 7]. Also, the dynamic variables are unreliable during cardiac surgery due to the open-chest condition [8].
The fundamental physiologic idea behind dynamic variables—a varying preload—appears to be the key when searching for reliable fluid responsiveness techniques, a notion supported by the results obtained with the passive leg raising (PLR) method [9]. However, PLR is not feasible in the operating room due to obvious reasons [10]. So, the challenge to overcome is to identify another preload changing mechanism. We have recently suggested a novel method that is based on the occurrence of extra systoles (ES). An ES is comprised by, first, the premature/ectopic beat with decreased preload, then, the post-ectopic beat with moderately increased preload due to the prolonged filling time (compensatory pause) [11]. Consequently, the post ectopic beat is associated with a right shift of Frank-Starling curve and may elucidate the hemodynamic effect of giving fluids. The method’s physiologic basis has been confirmed experimentally [12], in post-cardiac surgery patients [13], and in intensive care patients [14], but has not been tested perioperatively during cardiac surgery.
In addition to the ES method, a rapid (10 s), central infusion of 50 ml crystalloid, which we in the following term a micro-fluid challenge (micro-FC), has also recently been suggested as a possible transient preload change with promising results for predicting fluid responsiveness when measuring the micro-FC response in left ventricular outflow tract (LVOT) blood flow velocity–time integrals (VTIs) measured with echocardiography [15].
The present study aimed at investigating the ES method during cardiac surgery, and we hypothesised that the post-ectopic beat characteristics, when compared with preceding sinus beats, could predict fluid responsiveness following a 5 ml/kg fluid challenge. As a secondary aim of the study, we investigated whether a micro-FC given as the first part of the fluid challenge could predict fluid responsiveness by measuring responses in arterial blood pressure (ABP) characteristics and VTIs.
2 Methods
This prospective study was approved by the local Institutional Review Board (METc UMCG number 2016.449; ABR number NL58966.042.16) and the protocol was submitted to ClinicalTrials.gov (NCT03002129). All patients gave written informed consent prior to surgery. The study is reported according to STARD guidelines [16]. This paper contains supplemental digital content.
2.1 Patients
Patients aged ≥ 18 years scheduled for elective coronary artery bypass graft (CABG) surgery with no accompanying surgical procedures were consecutively screened for eligibility. Patients with preoperative left ventricular ejection fraction (LVEF) less than 35%, kidney function requiring haemodialysis, chronic atrial fibrillation or frequent/coupled extra systoles (e.g. trigemini) were excluded.
2.2 Study protocol
Patients were anaesthetised according to our institution’s local protocols (target controlled infusions of propofol and sufentanil [17]) and equipped with standard monitoring (ECG, continuous ABP via radial catheter, CVP via jugular venous catheter, pulse oximetry). Furthermore, patients were equipped with FloTrac® sensors connected to EV1000 monitors (both Edwards Lifesciences, Irvine, USA) for continuous stroke volume (SV) and cardiac output (CO) measurements.
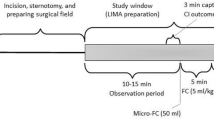
The study intervention consisted of two lactated Ringer (Baxter, Utrecht, the Netherlands) fluid challenges (5 ml/kg ideal body weight; ideal body weight estimated as height (in cm) − 100 for males and height − 105 for females) during the first perioperative phase of CABG surgery. Each fluid challenge was preceded by a 10–15 min observation window allowing spontaneous extrasystoles to occur prior to the fluid challenge. The approximate time line is illustrated in Fig. 1. The first window was initiated after induction of anaesthesia and insertion of the central venous catheter and ended no later than surgical incision. The second window was initiated at the onset of surgical preparation of the left internal mammarian artery (LIMA) and ended no later than the pericardial opening. In few cases, the pericardium was opened for inspection prior to LIMA preparation. In those cases, the LIMA preparation period still defined the 2nd study window. Both windows were coordinated with the surgeon to minimize surgical delay, so observation periods varied in length. The first 50 ml of the 5 ml/kg fluid challenge was infused rapidly on the largest central venous catheter lumen in approx. 10 s (micro-FC). The remaining fluid challenge infusion was initiated approx. 20 s later via a peripheral line in approx. five minutes using a pressure bag. Changes in infusion rates of vasoactive or inotropic drugs and changes in bed position were avoided and – if deemed necessary for patient treatment - precluded the recordings.
2.3 Data acquisition
ECG, ABP, CVP, and pulse oximetry waveforms were digitized at 125 Hz and captured using Rugloop II® software (Demed Engineering, Temse, Belgium). SV was updated every 20 s by the FloTrac/EV1000 system and exported in Excel format from the monitor for offline analyses. Both systems were given exact time stamps (with simultaneous button presses) for the relevant actions during the study windows to align timing. A transoesophageal echocardiography (TOE) probe was inserted after induction of anaesthesia and connected to a Vivid-E9® or Vivid-S6®, (General Electrics, Chicago, USA). Trans-gastric long axis view or deep trans-gastric five chamber view were used to measure VTIs using pulsed-wave Doppler (PWD). VTIs from three respiratory cycles were measured with TOE before the micro-FC, during/after micro-FC, and after the full 5 ml/kg infusion [15].
Preoperative demographics, medication and comorbidity were extracted from electronic health records. Pre-operative LVEF evaluated without a specific quantification but with a semi-quantitative “normal/good function” description was considered to have a normal ejection fraction of 60%.
2.4 Data analyses
Waveforms were exported from Rugloop to Matlab data format (Mathworks inc. Natick, USA) and filtered and upsampled from 125 Hz to 1000 Hz using zero padding as previously conceptually described [18]. Automatic R-spike detection in filtered and upsampled ECGs were carried out with KubiosHRV (University of Eastern Finland, Kuopio, Finland, version 2.2) that also allowed manual corrections of the automatic detection (e.g. in case of electrical noise from electrocautery). The visually confirmed R spike detections were used for subsequent signal processing and feature extraction done in Matlab. Extrasystoles in the observation periods were identified and evaluated for eligibility defined by coupling interval of 80% or less than the average RR interval of the 10 preceding heart beats, which had to be sinus beats defined by none of these 10 preceding heart beats having an RR interval 10% higher or lower than any of the nine other heart beats. In addition, the post-ectopic RR interval (compensatory pause) had to be at least 5% higher compared to the preceding sinus beat RR interval. Post-ectopic changes in systolic blood pressure (SBP), pulse pressure (PP), pre-ejection period (PEP), and maximal systolic upstroke slope (dP/dt) were calculated as previously described [13].
For the micro-FC, a baseline period was defined as the 30 s preceding micro-FC initiation, and the micro-FC effect was determined from the 30 s following initiation of the fast infusion. SBP, PEP, PP, and dP/dt were determined for each heartbeat. The median of these variables from the baseline period and the 75-percentile from the 30 s during/after micro-FC were calculated to capture a possible increase in the variables as visual inspection revealed a varying timing of the peak of these variables, and variables were calculated in this scheme exemplified by SBP:
During automated offline detections in ABP and ECG, the programmer/assessor (STV) was blinded to the outcome.
VTI was averaged over three respiratory cycles, and the VTI response (∆VTI) to the micro-FC was calculated as the change from before micro-FC to the 15 s during and immediately after the micro-FC as previously described [15]. The assessor of VTI recordings (MM) was blinded to the outcome (fluid response defined by EV1000 data).
PPV and PEP variation (PEPV) were derived from the 30 s before onset of the micro-FC as previously described [18].
During data analysis, a transient micro-FC induced increase in RR intervals (i.e. drop in heart rate) was (unexpectedly) identified in most patients and therefore quantified as the difference between the median RR interval 10 s before infusion and the 90th percentile of the RR interval 30 s during/after micro-FC. The RR interval change was analysed for its fluid responsiveness prediction ability and it was also used as a correcting factor to the ABP derived micro-FC predictors (relative changes in SBP, PEP, PP, and dP/dt) assuming that an RR interval change also reflected a transient change in cardiac contractile state counteracting a preload induced change in the ABP predictors. As such, we calculated RR interval corrected variables according to the following scheme, exemplified by SBP:
2.5 Classification and statistics
Fluid responsiveness was defined as a SV change of 10% or more from before to after the 5 ml/kg fluid challenge. Averages of three consecutive FloTrac/EV1000® SV estimates (each derived from 20 s) before and after 5 ml/kg infusion were the basis of the SV change calculation: Fluid response = 100%*(SVafter – SVbefore)/SVbefore. After complete hemodynamic offline analyses, predictors and outcomes were merged (by STV) to calculate predictive ability. Based on previous data [13], we calculated sample size for the primary study outcome (the extra systoles method), assuming equal numbers of fluid responders and non-responders. Using a significance level of 0.05 and power of 0.9, we needed 30 patients with extra systoles and assuming that about half of patients had one or more extra systoles during at least one of the two planned study windows, we aimed at including 60 patients. Summary statistics are presented as mean (standard deviation) unless otherwise indicated. Paired t test was used to compare baseline hemodynamics with hemodynamics after volume expansion.
All statistics were performed with ‘R’ including statistics for ROC curve areas that were made with DeLong method implemented (R studio version 3.2.3, using package ‘pROC’ released 2015-05-04). Sensitivity, specificity and optimal threshold were reported using Youden Index and only for ROC area point estimates above 0.5. Data are reported as mean (standard deviation) if not otherwise indicated. p < 0.05 is considered statistically significant.
3 Results
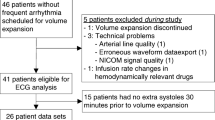
Patient inclusion is shown in Fig. 2.
Demographics and clinical characteristics for the entire cohort (n = 61) are shown in Table 1. The 5 ml/kg FCs corresponded to 376 (47) ml.
PPV and PEPV were quantifiable for 35 and 33 subjects, respectively, in the first window and for 52 and 48 subjects in the second window (exclusions were due to ABP noise artefacts considered to significantly influence maximal and minimal value estimates over respiratory cycles, where PEP is more sensitive to artefacts). The study windows for ES inspection were on average 13.2 min. There were fewer windows available (n = 37 vs n = 59) and a tendency to shorter duration (mean of 12.1 vs. 13.8 min) in the first window compared with the second window.
In the first study window, 21 of 37 (57%) analysed patients were fluid responders and in the second window, 25 of 54 (46%) analysed patients were fluid responders. Hemodynamic characteristics before and after the 5 ml/kg FC is shown in Table 2. On the group level, HR fell, MAP was unchanged and SV increased with the FC. Norepinephrine was infusing (without rate changes) in seven out of 37 analysed patients in the first window and 18 out of 59 analysed patients in the second window.
Classification of fluid responsiveness (scatter plots with corresponding ROC curves) is presented in Figs. 3 and 4 and S1-2 for the ES method and the micro-FC method for both study windows. For the ES method, ROC curve areas (AUC) for predicting fluid responsiveness ranged from 0.56 to 0.69 in the first window, where 15 patients (41% of analysed ECGs) had eligible ESs, and from 0.50 to 0.65 in the second window, where 27 patients (46% of analysed ECGs) had eligible ESs. For the micro-FC method, AUCs from ABP derived characteristics ranged from 0.59 to 0.74 in the first window (n = 37) and from 0.56 to 0.62 in the second window (n = 54). For logistic and echocardiographic quality reasons, micro-FC induced ∆VTI was possible to estimate in 14 cases in the first study window and in 32 cases in the second window. ∆VTI predicted fluid responsiveness with AUCs of 0.38 and 0.45 at the first and second micro-FC, respectively, see Fig. S3. Classification with PPV and PEPV are presented in supplemental Figs. S4 and S5. PPV and PEPV had AUCs of 0.64 and 0.68 in the first window and 0.56 and 0.40 in the second window. Among the patients where PPV was quantifiable, tidal volume indexed to predicted body weight (according to the Acute Respiratory Distress Network formula) was 7.1 (± 0.7) in the first window and 6.8 (± 0.8) in the second window. HR-to-Respiratory-Rate (HR/RR) ratio was 4.0 (± 0.9) in the first window and 3.8 (± 0.9) in the second window, see Table 2. Indexing PPV and PEPV to tidal volume [19] did not improve classification.
RR intervals increased immediately by 6.3 (± 4.6) % with the micro-FC in the first window and by 6.8 (± 4.9) % in the second window, see supplemental Figs. S6 and S7. The micro-FC induced increase in RR intervals (i.e. drop in heart rate) had AUCs of 0.76 and 0.61 for predicting fluid responsiveness in the first and second windows, respectively, see Fig. S8. RR interval corrected ABP variables (during micro-FC) had AUCs from 0.76 to 0.84 in the first window and 0.62 to 0.66 in the second window, see Figs. S9 and S10 and Table 3. VTI classification was not improved when corrected for RR interval changes.
Detailed classification characteristics including sensitivities and specificities are presented for each study window in Table 3.
4 Discussion
The ES method predicted fluid responsiveness with poor to mediocre accuracy (AUC point estimates ranging from 0.5 to 0.69) and can therefore not be recommended for individual use during cardiac surgery. This stands in contrast to previously published clinical data obtained postoperatively in a similar population [13] and in intensive care patients [14]. From a physiological viewpoint, the baseline heart rates could explain the reduced predictive power: Heart rates were much lower in this study compared with the previous study and it is possible that the compensatory pause was not causing a reliable preload increase in all subjects in this study. This may be related to beta-blocker use. Considering physiology, there may also be other explanations for the divergence in terms of classification accuracy, as we in this study used a fluid challenge of slightly less volume (376 ml compared with 500 ml). We might not create as strong a “response signal” (SV change), whose definition of a positive-response was set at 10% or higher for the same reason, another difference comparing with previous research on the method [13, 14]. From a practical viewpoint, we investigated patients that were constantly “advancing” in their anaesthetic and/or surgical procedure and therefore in a somewhat dynamic environment in both study windows, although the patients were anaesthesiologically and hemodynamically stable. This could reduce the predictive value of ESs that occurred early in the study windows, i.e. relatively long time before FC onset. Also, the fact that we did not detect 30 patients but only 27 with ES in the last study window means that we did not reach the assumed statistical power. Three additional data points could have narrowed confidence intervals for the ROC curve areas to become significantly different from 0.5 for study window 2 (which the power calculation was for), but this is unlikely to alter our overall study conclusions that the ES method is not demonstrating clinically adequate fluid responsiveness prediction. Finally, from a technical viewpoint, we used another SV monitoring modality in this study (fourth generation FloTrac compared with thermodulition continuous cardiac output or non-invasive cardiac output in previous studies [13, 14]). The trending ability of fourth generation FloTrac is reportedly around 76–88% [20, 21] (concordance rates), which is fair but not excellent concordance rates and could contribute to the weak classification results obtained for the ES method in this study.
Still, the reason for a poor ES method classification is most likely that the ES method simply does not have adequate predictive abilities in this perioperative setting of cardiac surgery, and this appears the case whether or not the thorax is open.
The micro-FC demonstrated better but still not optimal accuracy in predicting fluid responsiveness. It may be that the infusion amount of only 50 ml is not sufficient to alter preload transiently. In that regard, more compelling classification characteristics have been reported for the mini-fluid challenge (100 ml) [22]. Still, compelling results with infusion of only 50 ml has also been reported in relation with VTI change measures. ∆VTI had poor predictive ability in this study, which is surprising considering the otherwise promising results of a previous study [15]. The AUC point estimates in our study were even < 0.5 suggesting that low ∆VTI levels should indicate a positive fluid response. The major difference between our and the previous study is the monitoring modality for the outcome. We used the FloTrac/EV1000 system, whereas the authors of the previous study used transthoracic echocardiography (TTE). This monitoring modality was the same for outcome (VTI and CO) and predictor (∆VTI), which should be kept separated for optimal methodology [23]. Also, we used TOE to measure LVOT VTI, whereas the previous study used TTE [15].
The PPV and PEPV methods, which have shown excellent predictive abilities in ICU patients, performed poorly, also during closed thorax conditions. The poor classifications also during closed thorax may be due to ventilator settings (relatively low tidal volumes and HR/RR ratios). It is unusual to find PPV and PEPV with similar and low power of prediction of fluid responsiveness, if we compare the present study’s results with the first studies demonstrating the concept of PEPV [24, 25], and also when comparing with a subsequent study, where low tidal volumes were applied [26]. It therefore seems likely that a relatively low HR/RR ratio (also present during closed-thorax conditions in the present study) could contribute to the weak ability of PPV and PEPV to predict fluid responsiveness in the present study. The fact that HR was lower in this study compared with previous research could be in favor of making systolic interval times (e.g. PEPV) in a better posture than PPV. However, we neither found a better predictive power for PEPV compared with PPV, nor did we identify significantly better prediction among other PEP related variables compared with PP related variables. Overall, the AUC point estimates of the methods studied confirm that fluid responsiveness is hard to predict during open thorax surgery.
The micro-FC induced increase in RR interval was not anticipated by the authors but this characteristic had an AUC of 0.76 for predicting fluid responsiveness before incision. Patients that were “heart rate responsive” to a fast central infusion of 50 ml room temperatured Ringer’s lactate (i.e. a significant, but transient drop in heart rate, see Figs. S6 and S7) were likely to be fluid responders. Furthermore, considering that this drop in heart rate (chronotropic state change) may also reflect changes in inotropic state, we corrected the micro-FC predictors for the RR interval increase to get the “sum” of the preload (micro-FC) and inotropic changes (RR change). We believe that this is a physiologically plausible approach, because the changes in RR intervals were probably a physiologic response to the fast infusion. Nonetheless, since this RR interval correction is a coincidental study result, these findings can only be regarded as hypothesis generating, and even if the findings could be replicated and clinically useful fluid responsiveness prediction can be demonstrated, these interesting but preliminary results appear only to apply to closed thorax conditions in this study. As a final comment about this transient change in RR intervals following/during micro-FC, this may pose a question to thermodilution cardiac output (CO) calibration with infusion of colder but less amounts of fluids, because the heart beats with altered RR intervals appear to be the same heart beats responsible for the thermodilution curve (see Figs. S6 and S7). While outside the scope of the present study, this may be a physiologic mechanism to investigate further for bolus injection calibration of CO.
In conclusion, the present study revealed insufficient validity of novel alternative methods for predicting fluid responsiveness during cardiac surgery, particularly during open-thorax conditions, that remains an unsettled matter in terms of fluid responsiveness prediction. However, RR interval corrected changes during a micro-FC should be investigated further, since such variables provided good prediction of fluid responsiveness in this study in closed-thorax conditions.
Data availability
The dataset used and analyzed in the study is available from the corresponding author on request.
References
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005;103:419–28.
Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–8.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–7. https://doi.org/10.1097/CCM.0b013e3181a590da.
Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: how many shades of grey? Crit Care. 2015;19:144-015-0869-x. https://doi.org/10.1186/s13054-015-0869-x.
Maguire S, Rinehart J, Vakharia S, Cannesson M. Technical communication: respiratory variation in pulse pressure and plethysmographic waveforms: intraoperative applicability in a North American academic center. Anesth Analg. 2011;112:94–6. https://doi.org/10.1213/ANE.0b013e318200366b.
Cannesson M, Pestel G, Ricks C, Hoeft A, Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011;15:R197. https://doi.org/10.1186/cc10364.
Piccioni F, Bernasconi F, Tramontano GTA, Langer M. A systematic review of pulse pressure variation and stroke volume variation to predict fluid responsiveness during cardiac and thoracic surgery. J Clin Monit Comput. 2017;31:677–84. https://doi.org/10.1007/s10877-016-9898-5.
Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–83. https://doi.org/10.1007/s00134-010-1929-y.
Monnet X, Teboul JL. (2015) Passive leg raising: five rules, not a drop of fluid! Crit Care 19:18-014-0708-5. https://doi.org/10.1186/s13054-014-0708-5.
Cohn K, Kryda W. The influence of ectopic beats and tachyarrhythmias on stroke volume and cardiac output. J Electrocardiol. 1981;14:207–18.
Vistisen ST, Andersen KK, Frederiksen CA, Kirkegaard H. Variations in the pre-ejection period induced by ventricular extra systoles may be feasible to predict fluid responsiveness. J Clin Monit Comput. 2014;28:341–9. https://doi.org/10.1007/s10877-013-9528-4.
Vistisen ST. Using extra systoles to predict fluid responsiveness in cardiothoracic critical care patients. J Clin Monit Comput. 2017;31:693–9. https://doi.org/10.1007/s10877-016-9907-8.
Vistisen ST, Krog MB, Elkmann T, Vallentin MF, Scheeren TWL, Solling C. (2018) Extrasystoles for fluid responsiveness prediction in critically ill patients. J Intensive Care 6:52-018-0324-6. eCollection 2018. https://doi.org/10.1186/s40560-018-0324-6.
Wu Y, Zhou S, Zhou Z, Liu B. A 10-second fluid challenge guided by transthoracic echocardiography can predict fluid responsiveness. Crit Care. 2014;18:R108. https://doi.org/10.1186/cc13891.
Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799-2016-012799. https://doi.org/10.1136/bmjopen-2016-012799.
Absalom AR, Glen JI, Zwart GJ, Schnider TW, Struys MM. Target-controlled infusion: a mature technology. Anesth Analg. 2016;122:70–8. https://doi.org/10.1213/ANE.0000000000001009.
Vistisen ST, Koefoed-Nielsen J, Larsson A. Automated pre-ejection period variation predicts fluid responsiveness in low tidal volume ventilated pigs. Acta Anaesthesiol Scand. 2010;54:199–205. https://doi.org/10.1111/j.1399-6576.2009.02061.x.
Vistisen ST, Koefoed-Nielsen J, Larsson A. Should dynamic parameters for prediction of fluid responsiveness be indexed to the tidal volume? Acta Anaesthesiol Scand. 2010;54:191–8. https://doi.org/10.1111/j.1399-6576.2009.02114.x; 10.1111/j.1399-6576.2009.02114.x.
Kusaka Y, Ohchi F, Minami T. Evaluation of the fourth-generation FloTrac/Vigileo system in comparison with the intermittent bolus thermodilution method in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2018. https://doi.org/10.1053/j.jvca.2018.06.017.
Suehiro K, Tanaka K, Mikawa M, et al. Improved performance of the fourth-generation FloTrac/Vigileo system for tracking cardiac output changes. J Cardiothorac Vasc Anesth. 2015;29:656–62. https://doi.org/10.1053/j.jvca.2014.07.022.
Guinot PG, Bernard E, Defrancq F, Petiot S, Majoub Y, Dupont H, Lorne E. Mini-fluid challenge predicts fluid responsiveness during spontaneous breathing under spinal anaesthesia: An observational study. Eur J Anaesthesiol. 2015;32:645–9. https://doi.org/10.1097/EJA.0000000000000175.
Vistisen ST, Scheeren TWL. Challenge of the mini-fluid challenge: filling twice without creating a self-fulfilling prophecy design. Anesthesiology. 2018;128:1043–4. https://doi.org/10.1097/ALN.0000000000002141.
Feissel M, Badie J, Merlani PG, Faller JP, Bendjelid K. Pre-ejection period variations predict the fluid responsiveness of septic ventilated patients. Crit Care Med. 2005;33:2534–9.
Bendjelid K, Suter PM, Romand JA. The respiratory change in preejection period: a new method to predict fluid responsiveness. J Appl Physiol. 2004;96:337–42. https://doi.org/10.1152/japplphysiol.00435.2003.
Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53:534–42. https://doi.org/10.1111/j.1399-6576.2008.01893.x.
Funding
STV was financially supported by The Danish Medical Research Council (DFF – 4183-00540) and the Danish Society for Anaesthesia and Intensive Care Medicine.
Author information
Authors and Affiliations
Contributions
STV: Conception of study, study design, data collection, data analysis, interpretation of data, writing up of the first draft of the paper, revising it critically for important intellectual content, final approval of the version to be published. JMB: Conception of study, study design, data collection, revising the paper critically for important intellectual content, final approval of the version to be published. MB: Patient recruitment, data collection, revising the paper critically for important intellectual content, final approval of the version to be published. MM: Study design, data collection, data analysis, revising the paper critically for important intellectual content, final approval of the version to be published. RB: Study design, data collection, revising the paper critically for important intellectual content, final approval of the version to be publishedJSJ: Study design, data collection, revising the paper critically for important intellectual content, final approval of the version to be published. MAM: Study design, revising the paper critically for important intellectual content, final approval of the version to be published. TWLS: Conception of study, study design, data collection, interpretation of data, writing up of the first draft of the paper, revising it critically for important intellectual content, final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
STV: The extrasystoles method was previously protected by a patent application owned by Aarhus University (PCT/DK2014/050094; STV as sole inventor). The application did, however, not lead to granting of a patent at priority expiry date. MAM has received honoraria for consulting and training activities for LivaNova. MAM has received grants from Atricure, Edwards Lifesciences and Abbot. TWLS is associate editor of the Journal of Clinical Monitoring and Computing. TWLS received honoraria for consulting from Edwards Lifesciences and Masimo Corp. TWLS is currently Chair of the Section Cardiovascular Dynamics of the European Society of Intensive Care Medicine as well as Chair of the Scientific Subcommittee 14 (Monitoring, Ultrasound and Equipment) of the European Society of Anaesthesiology.JMB, MB, MM, RB, and JSJ report no conflicts of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vistisen, S.T., Berg, J.M., Boekel, M.F. et al. Using extra systoles and the micro-fluid challenge to predict fluid responsiveness during cardiac surgery. J Clin Monit Comput 33, 777–786 (2019). https://doi.org/10.1007/s10877-018-0218-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0218-0